Synthesis and Characterization of Complex Compounds from Cadmium(II) Chloride and Cobalt(II) Chloride with N,N'-Diethylthiourea
Abstract
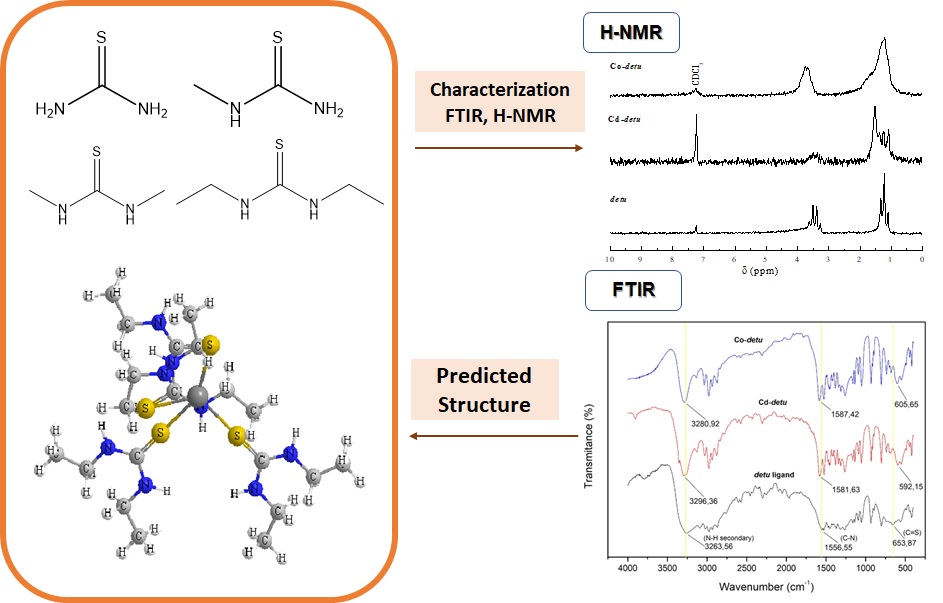
The synthesis of ionic complexes of cadmium(II) chloride and cobalt(II) chloride with N,N'-diethylthiourea (detu) ligands has not been previously reported. Therefore, in this research, the synthesis was carried out to study the structure and characterization of the two ionic complex compounds. The cadmium(II)-detu ionic complex was synthesized using the direct reaction method with a ratio between Cd(II) salt and detu ligand of 1:2. Meanwhile, the cobalt(II)-detu ionic complex was synthesized with a ratio between Co(II) salt and detu ligand of 2:4. The cadmium(II)-detu and cobalt(II)-detu ionic complexes have melting points of 105-108 ˚C and 122-125 ˚C, respectively. The electrical conductivity of the cadmium(II)-detu and cobalt(II)-detu complexes showed that the complexes were ionic. The FTIR analysis showed the shifting of the C=S functional group’s band to the smaller wavenumber, which indicates the coordinating detu ligand to the cadmium(II) and cobalt(II) through the S atom. The indirect evidence from 1H-NMR showed that CH3 and CH2 only slightly shifted between the free detu ligand and the Cd-detu and Co-detu complexes.
References
[1] Rahman, F. U., Bibi, M., Altaf, A. A., Tahir, M. N., Ullah, F and Khan, E., J. Mol. Struct, 2020, 1211, 128096.
[2] Anacona, J. R and Rodriguez, I., J. Coord. Chem, 2004, 57 (15), 1263-1269.
[3] Golcu, A., Tumer, M., Demirelli, H and Wheatley, R. A., Inorganica Chim. Acta, 2005, 358 (6), 1785-1797.
[4] Montazerozohori, M., Zahedi, S., Nasr-Esfahani, M and Naghiha, A., J. Ind. Eng. Chem, 2014, 20 (4), 2463-2470.
[5] Sönmez, M., Berber, İ and Akbaş, E, Eur. J. Med. Chem, 2006, 41 (1), 101-105.
[6] Khadivi, R., Pasdar, H., Foroughifar, N and Davallo, M., Int. J. Adv. Eng. Manag. Sci, 2018, 4 (9), 264324.
[7] Zhao, H. Y., Ma, J. J., Yang, X. D and Yang, M. L, Synth. React. Inorganic, Met. Nano-Metal Chem, 2016, 46 (1), 45-50.
[8] Atakilt, A., Bayissa, G., Sendek, A and Kibret, M, Ethiop. J. Sci. Technol, 2018, 11 (2), 79-96.
[9] Fariati., Dasna, I. W., Fadli, Y. F and Qurbayni, S. H, “Study of structure prediction of complex compounds of zinc(II) chloride and cadmium(II) chloride with potassium cyanide and N,N’-diethylthiourea ligand,” in AIP Conference Proceedings, 2020, 2251, 1, 040038.
[10] Pearson, R. G, J. Am. Chem. Soc., 1963, 85 (22), 3533–3539.
[11] Jacobson, K. B and Turner, J. E, Toxicology, 1980, 16 (1), 1-37.
[12] Ajibade, P. A., Zulu, N. H and Oyedeji, A. O, Synth. React. Inorganic, Met. Nano-Metal Chem, 2013, 43 (5), 524-531.
[13] Mohapatra, R. K et al., Comments Inorg. Chem, 2019, 39 (3), 127–187.
[14] Marcotrigiano, G, Z. Anorg. Allg. Chem, 1976, 422 (1), 80-88.
[15] Ahmad, S., Amir, Q., Naz, G., Fazal, A., Fettouhi, M., Isab, A. A., Rüffer, T and Lang, H, J. Chem. Crystallogr, 2012, 42(6), 615-620.
[16] Ahmad, S., Saleem, M., Georgieva, I., Ruffer, T., Schaarschmidt, D., Lang, H., Murtaza, G., Hussain, I., Isab, A. A., Malik, M. R and Ali, S, Polyhedron, 2018, 149,126-133.
[17] Ajibade, P. A, Sci. World J, 2013, 2013, 6.
[18] Ahmad, S., Fettouhi, M., Roisnel, T., Alotaibi, M. A., Alharthi, A.I., Malik, M.R., Ahmad, I. and Isab, A.A, J. Coord. Chem, 2017, 70 (21), 3692-3701.
[19] Altaf, M., Stoeckli-Evans, H., Murtaza, G., Isab, A. A., Ahmad, S and Shaheen, M. A J. Struct. Chem, 2011, 52 (3), 625-630.
[20] Mahmood, R., Hussain, S.G., Isab, A.A., Fettouhi, M., Fazal, A and Ahmad, S., J. Struct. Chem., 2015, 56 (3), 463-467.
[21] Fawcett, T. G., Fehskens, E. E., Potenza, J. A., Schugar, H. J and Lalancette, R. A., Acta Crystallogr. B, 1979, 35 (6),1460–1463.
[22] Askalani, P., Bailey, R. A., Itlstitute, R. P and Arab, U., Meas. Tech, 1969., 47, 2275-2282.
[23] Malik, M.R., Rüffer, T., Lang, H., Isab, A. A., Ali, S., Ahmad, S and Stoeckli-Evans, H., J. Struct. Chem., 2013., 54 (4), 810-814.
[24] Ozturk, I.I et al., Inorganica Chim. Acta, 2019, 491, 14-24.
[25] Jambi, S. M, J. Mol. Liq, 2018, 262, 237-247.
[26] Elhusseiny, A. F., Eldissouky, A., Al-Hamza, A. M and Hassan, H. H, J. Mol. Struct, 2015, 1100, 530-545.
[27] Binzet, G., Arslan, H., Flörke, U., Külcü, N and Duran, N, J Coord Chem, 2006, 59 (12), 1395-1406.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.