A Comparative Study on The Adsorption Behavior of Congo Red on to ZnAl and ZnCr Layered Double Hydroxides
Abstract
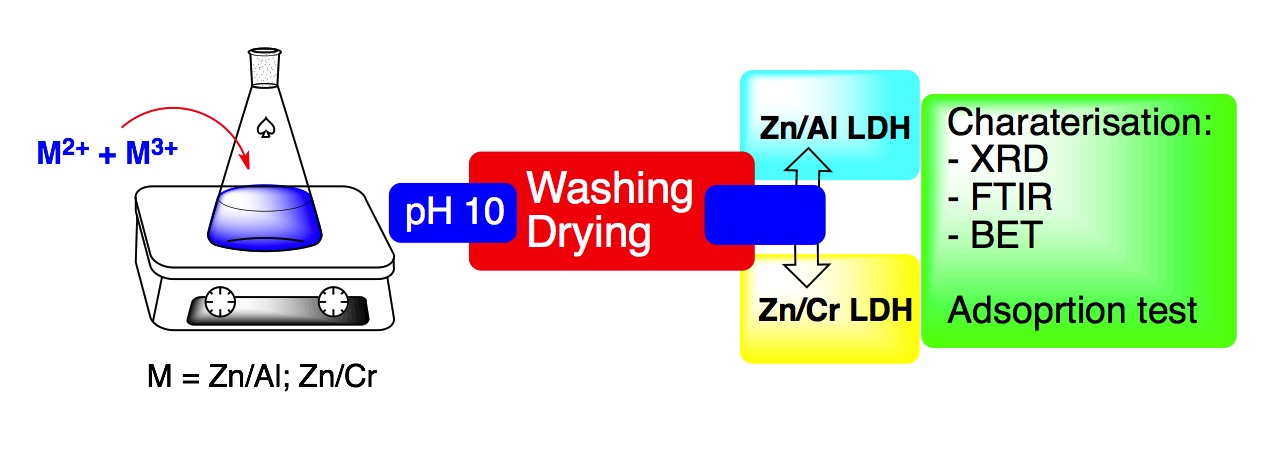
References
[1] Mohapatra, L., and Parida, K.M, Sep. Purif. Technol, 2012, 91, 73-80.
[2] Narvekar, A.A., Fernandes, J.B., and Tilve, S.G, J. Environ. Chem. Eng, 2018, 6(2), 1714-1725.
[3] Taher, T., Rohendi, D., Mohadi, R., and Lesbani, A, Chiang Mai J. Sci, 2018, 45(4), 1770-1781.
[4] Li, Y., Meas, A., Shan, S., Yang, R., and Gai, X, Bioresour. Technol, 2016, 207, 379-386.
[5] Su, J., He, S., Zhao, Z., Liu, X., and Li, H, Colloid Surfaces A, 2018, 554, 227-236.
[6] Taher, T., Mohadi, R., and Lesbani, A, Clay. Miner, 2018, 15, 16.
[7] Palapa, N.R., and Said, M, Sci.Technol. Indones, 2017, 1(1), 25-28.
[8] Lin, S.T., Tran, H.N., Chao, H.P., and Lee, J.F, Appl. Clay Sci, 2018, 162, 443-453,
[9] Cocheci, L., Barvinschi, P., Pode, R., Seftel, E.M., and Popovici, E, Adsorp Sci Technol, 2010, 28(3), 267-279.
[10] Liu, J., Song, J., Xiao, H., Zhang, L., Qin, Y., Liu, D., Hou, W., and Du, N, Powder Technol, 2014, 253, 41-45.
[11] Hirata, N., Tadanaga, K., and Tatsumisago, M., Mater. Res. Bull, 2015, 62, 1-4.
[12] Pérez-Barrado, E., Salagre, P., Marsal, L.F., Aguilo, M., Cesteros, Y., Diaz, F., Pallares, J., Cucinotta, F., Marchese, L., and Pujol, M.C, Appl. Clay Sci, 2015, 118, 116-123.
[13] Tao, X., Liu, D., Cong., and Huang, L, Appl Surf Sci, 2018, 457, 572-579.
[14] Ma, L., Zhao, G., Fang, Y., Dai, W., and Ma, N, Adsorp Sci Techn, 2018, 36(3-4), 872-887.
[15] I. Langmuir, "The Adsorption Of Gases On Plane Surfaces Of Glass, Mica And Platinum," J. Am. Chem. Soc, 1918, 40(9), 1361-1403.
[16] Freundlich, H., Z Phys.Chem (N F), 1906, 62(5), 121-125.
[17] Munagapati, V.S., and Kim, D.S, Ecotoxicology and Environmental Safety, 2017, 181-193..
[18] Mohebali, Bastani, D., and Shayesteh, S., J Mol. Structure, 2018, 1176, 181-193
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








