Synthesis of Eugenyl Cinnamate from Clove Oil (Syzygium aromaticum) via Bromination-Dehydrobromination Methods
Abstract
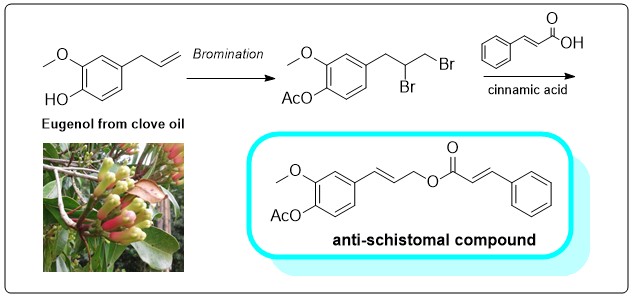
Synthesis of bioactive materials based on Indonesian natural products as precursors are potential to achieve a sustainable supply of modern medicines. Eugenyl cinnamate is a crucial building blocks in many bioactive compunds such as hepatoprotective silibinin. This research features simple synthesis of eugenyl cinnamate from eugenol, an essential oil presents as major constituent of clove oil (Syzygium aromaticum). The transformations were carried out via protection of hydroxyl group, bromination, and dehydrobromination reactions of eugenol (1) consecutively. The products of the synthesis were purified by gravity column chromatography and were characterized by FTIR and NMR spectroscopy. Benzylation of eugenol was carried out under basic condition with high yield (94.3%). Characterization by spectroscopic methods showed that eugenyl benzyl ether (2) was formed. Bromination of eugenyl benzyl ether yielded three products, namely: dibromo (3a and 3b), and tri-bromo eugenyl benzyl ether (3c). Compound 3a and 3b were epimers based on intensive NMR analysis (1H, 13C and DEPT). These epimers were separable using simple gravitational colomn chromatography. Bromination of eugenyl acetyl ether (4) yielded the targeted dibromo product (5). Dehydrobromination reaction of compound 5 with cinnamic acid yielded the eugenyl cinnamate (6) with yield of 23.2%. This compound is precursor in the hetero Diels-Alder reaction to form the dioxan unit of Silibinin.
References
(1) Blaser, H.-U., Malan, C., Pugin, B., Spindler, F., Steiner, H., Studer, M. Adv. Synth. Catal. 2003.
(2) Hakim, E. H., Achmad, S. A., Juliawaty, L. D., Makmur, L., Syah, Y. M., Aimi, N., Kitajima, M., Takayama, H., Ghisalberti, E. L. J. Nat. Med. 2006.
(3) Torres, A. I., Tsapatsis, M., Daoutidis, P. Comput. Chem. Eng. 2012.
(4) Cortés-Rojas, D. F., de Souza, C. R. F., Oliveira, W. P. Asian Pacific Journal of Tropical Biomedicine. 2014.
(5) rajatani.my.id. (2020). rajatani.my.id -. [online] Available at: http://www.rajatani.my.id/ [Accessed 12 Jan. 2020].
(6) Schümann, J., Prockl, J., Kiemer, A. K., Vollmar, A. M., Bang, R., Tiegs, G. J. Hepatol. 2003.
(7) Glaser, J., Schurigt, U., Suzuki, B. M., Caffrey, C. R., Holzgrabe, U. Molecules 2015.
(8) Briot, A., Baehr, C., Brouillard, R., Wagner, A., Mioskowski, C. Tetrahedron Lett. 2003.
(9) Fuhrmann, E., Talbiersky, J. Org. Process Res. Dev. 2005.
(10) Berti, G., Marsili, A. Tetrahedron 1966.
(11) Lim, C., Baek, D. J., Kim, D., Youn, S. W., Kim, S. Org. Lett. 2009.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.