Influence of Sulfonated-Kaolin On Cationic Exchange Capacity Swelling Degree and Morphology of Chitosan/Kaolin Composites
Abstract
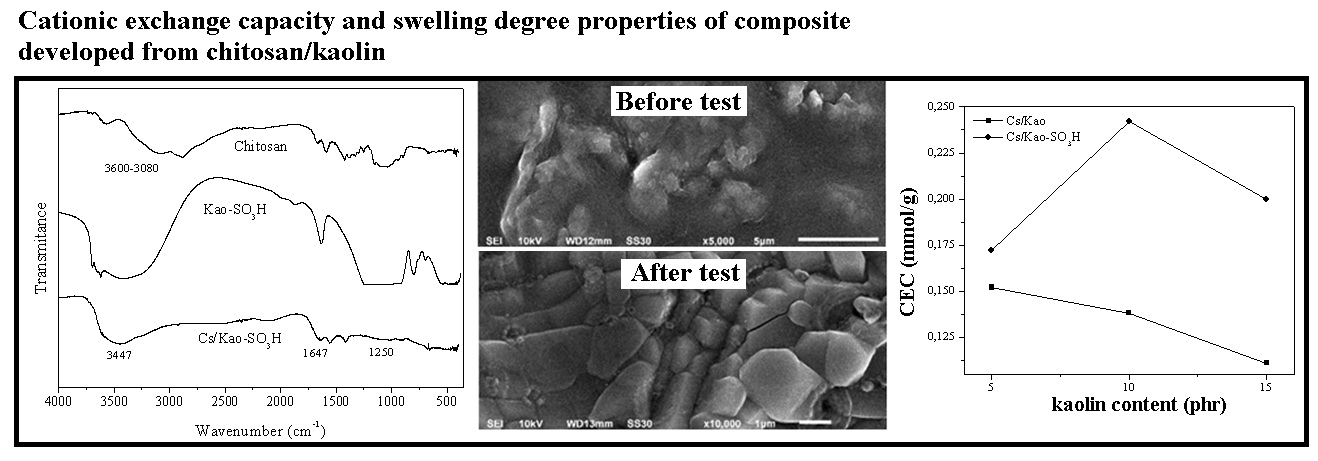
References
Peng, Y., Lu, H., Wang, Z. and Yan, Y., J. Mater. Chem. A., 2014, 2, 16093-16100.
Annadurai, G., Bioprocess Eng., 2000, 23, 451-455.
Kiakhani, M.S., Arami, M. and Gharanjig, K., J. Environ. Chem. Eng., 2013, 1, 406-415.
Mukoma, P., Jooste, B.R. and Volsloo, H.C.M., J. Power Sources., 2004, 136, 16-23.
He, Y., Tong, C., Geng, L., Liu, L. and Lu, C., J. Membrane Sci., 2008, 458, 36-46.
Ahmed, M. and Dincer, I., Int. J. Energ. Resour., 2011, 35, 1213-1228.
Mistri, E.A., Mohanty, A.K., Banerjee, S., Komber, H. And Voit, B., J. Membrane Sci., 2013, 441, 168-177.
Bai, H., Zhang, H., He, Y., Liu, J., Zhang, B. and Wang, J., J. Membrane Sci., 2014, 454, 220-232.
Ozer, O., Ince, A., Karagoz, B. and Bicak, N., Desalination., 2013, 309, 141-147.
Klyasom, C., Ladewig, B.P., Lu, G.Q.M. and Wang, L., J. Membrane Sci., 2011, 368, 48-53.
Cestari, A.R., Vieira, E.F.S., Tavares, A.M.G. and Bruns, R.E., J. Hazard. Mater., 2008, 153, 566-574.
Daraei, P., Madaeni, S.S., Salehi, E., Ghaemi, N., Ghari, H.S., Khadivi, M.A. and Rostami, E., J. Membrane Sci., 2013, 436, 97-108.
Park, S.S., Hwang, E.H., Kim, B.C. and Park, H.C., J. Am. Ceram. Soc., 2000, 83, 1341-1345.
Zhang, B., Li, Y., Pan, X., Jia, X. and Wang, X., J. Phys. Chem. Solid., 2007, 68, 135-142.
Olejnik, S., Posner, A.M. and Quirk, J.P., Clay Miner., 1970, 8, 421-434.
Valášková, M., Rieder, M., Matějka, V. and Čapková, P., Appl. Clay Sci., 2007, 35, 108-118.
Ismail, H., Khoo, W.S. and Ariffin, A., J. Vinyl Addit. Technol. DOI 10.1002/vnl.20331
Mohamed, N.S., Subban, R.H.Y. and Arof, A.K., J. Power Sources., 1995, 56, 153-156.
Wang, F., Hickner, M., Kim, Y.S., Zawodzinski, T.A. and McGranth, J.E., J. Membrane Sci., 2002, 197, 231-242.
Sugahara, Y., Satokawa, S., Yoshioka, K.I., Kuroda, K. and Kato, C., Clay Clay Miner., 1989, 37, 143.
Jimenez, M., Duquesne, S., and Bourbigot, S., Ind. Eng.
Chem. Res., 2006, 45, 4500-4508.
Haiyun M., Lifang T., Zhongbin X., and Zhengping F., Appl. Clay Sci., 2008, 42, 238-245.
Refbacks
- There are currently no refbacks.









