Synthesis of Microsheets Bi4Ti3O12 and Bi4Ti2.95V0.05O12 via Molten NaCl-KCl Salt Method
Abstract
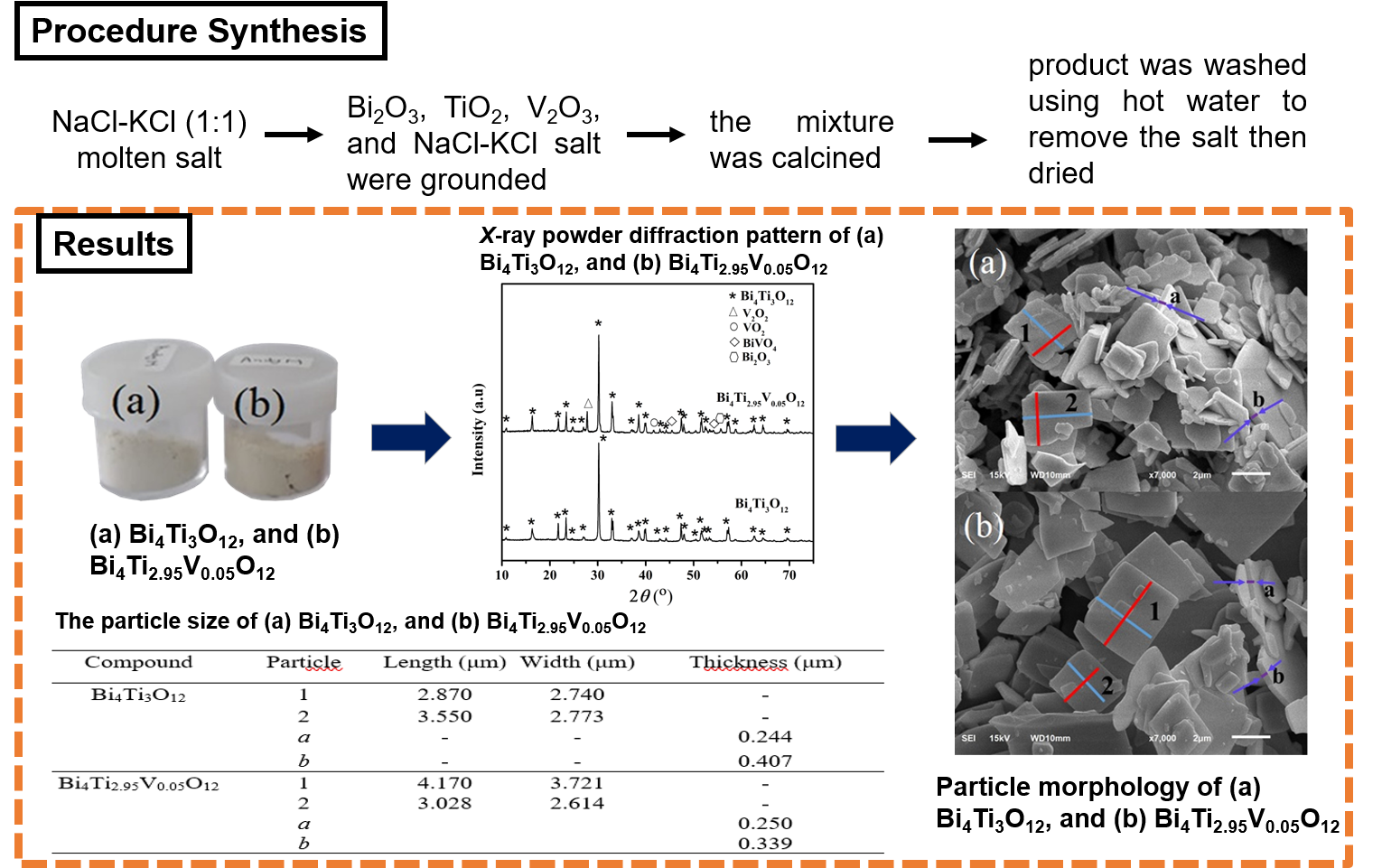
Bi4Ti3O12 is a tri-layer Aurivillius member compound that was reported to have good photocatalytic properties. Metal element doping and morphological particle tuning are strategies to increase photocatalyst activity. In this research, the compound micro sheets Bi4Ti3O12 and Bi4Ti2.95V0.05O12 were synthesized using molten NaCl/KCl salt. The diffractogram shows that the Bi4Ti3O12 sample was successfully synthesized, however, there are still found impurities at the Bi4Ti2.95V0.05O12 sample. Micrographs showed that the morphology particle samples is. The results of UV-Vis DRS spectra calculation show that both samples have a band gap energy of ~2.97 eV.
References
Forgacs, E., Cserhati, T., and Orosb, G., Environ. Int., 2004, 30, 953–971.
Anwer, H., Mahmood, A., Lee, J., Kim, K.H., Park, J.W., and Yip, A.C.K., Nano Res., 2019, 12, 5, 955-972.
Zhu, Z., Wan, S., Zhao, Y., Gu, Y., Wang, Y., Qin, Y., Zhang, Z., Ge, X., Zhong, Q., and Bu, Y., Materials Reports: Energy, 2021, 1, 100019.
Rouf, U.A., Hastuti, E., and Prasetyo, A., Jurnal Kartika Kimia, 2021, 4, 1, 51-57.
Aurivillius, B. Arkiv For Kemi, 1949, I, 54, 463-480.
Khan, M. A., Nadeem, M. A. and Idriss, H., Surf. Sci. Rep., 2016, 71, 1, 1–31.
Chen, P., Liu, H., Cui, W., Lee, S.C., Wang, L., and Dong, F., EcoMat, 2020, 2, 3, e12047
Wang, Y., Zhang, X., Zhang, C., Li, R., Wang, Y., and Fan, C., Inorg. Chem. Commun., 2020, 116, 107931.
Chen, Z., Jiang, X., Zhu, C., and Shi, C., Appl. Catal. B., 2016, 199, 241–251.
Liu, Y., Zhu, G., Gao, J., Hojamberdiev, M., Zhu, R., Wei, X., Guo, Q., and Liu, P., Appl. Catal. B., 2017, 200, 72-82.
Li, H., Zhao, G., Chen, Z., Han, G., and Song, B., J. Colloid Interface Sci., 2010, 344, 247–250.
Zhao, W., Jia, Z., Lei, E., Wang, L., Li, Z., and Dai, Y., J. Phys. Chem. Solids., 2013, 74, 1604-1607.
Chen, Z., Jiang, H., Jin, W., and Shi, C., Appl. Catal. B., 2016, 180, 698-706.
Kimura, T. 2011, Molten salt synthesis of ceramic powders. Advances in Ceramics Synthesis and Characterization, Processing and Specific Applications. Rijeka: In Tech.
Zhao, Z., Li, X., Ji, H., and Deng, M., Integr. Ferroelectr., 2014, 154, 54–158.
Januari T., Aini N., Barorroh H., Prasetyo A., IOP Conf. Ser.: Earth Environ. Sci. 2020, 456: 012013.
Marela, S.D., Aini, N., Hardian, A., Suendo, V., and Prasetyo, A., J. Pure App. Chem. Res., 2021, 10, 1, 64-71
Liu, Y., Zhu, G., Gao, J., Hojamberdiev, M., Zhu, R., Wei, X., Guo, Q., and Liu, P., Appl. Catal. B., 2017, 200, 72-82.
Shannon, R. D. 1976. Acta Crystallogr., 1976, A32, 751-767.
H. Maulidianingtiyas, A. D. Prasetiyo, F. Haikal, I. N. Cahyo, V. N. Istighfarini, and A. Prasetyo., Alchemy Jurnal Penelitian Kimia, 2021, 17, 2, 211-218.
Wang, Y., Zhang, X., Zhang, C., Li, R., Wang, Y., and Fan, C., Inorg. Chem. Commun., 2020, 116, 107931.
Lardhi, S., Noureldine, D., Harb, M., Ziani, A., Cavallo, L., and Takanabe, K., J. Chem. Phys., 2016, 144, 134702.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








