Synthesis of Struvite Crystal (MgNH4PO4.6H2O) from Laundry Waste to Consider its Potential as a Plant Fertilizer: Stirring and Processing Temperature Effect
Abstract
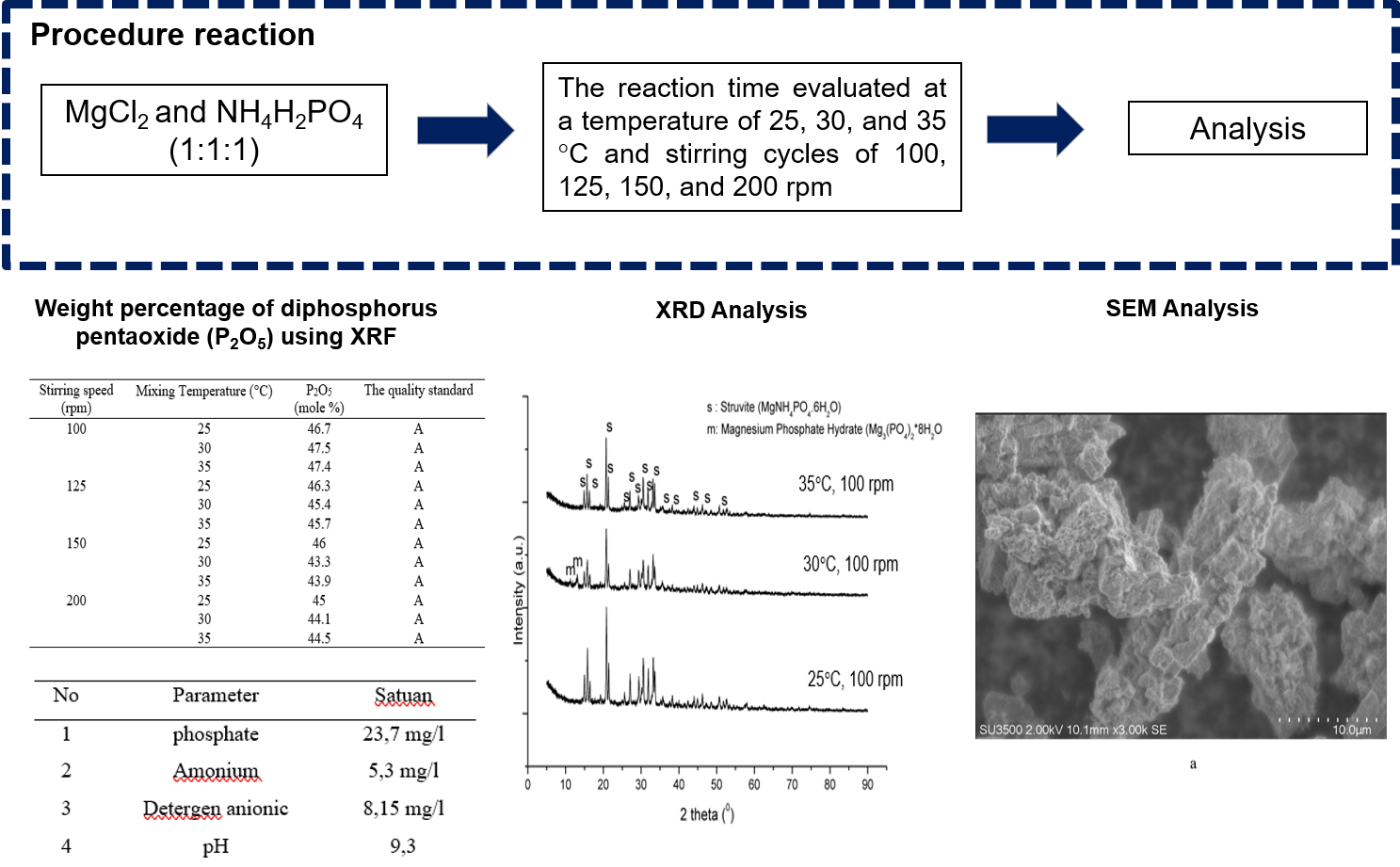
This paper aimed to process phosphate from laundry waste to be struvite crystal by precipitation and crystallization methods. In general, phosphates are difficult to remove by conventional treatment technologies. Therefore, precipitation and crystallization methods can be an alternative choice for phosphate recovery. Precipitation and crystallization methods can serve to remove dissolved phosphate content in wastewater, as well as convert it into a solid form that can be reused as industrial raw materials. The research variables used were stirring temperature (25, 30, and 35 °C) and stirring speed (100, 125, 150, and 200 rpm). By using X-ray Fluorescence (XRF) and Scanning Electron Microscope (SEM-EDX), the analysis showed that the highest percentage of phosphate removal was at a stirring speed of 100 rpm at 30°C, which was 47.5%. X-ray Diffraction (XRD) was also carried out on several samples and it was confirmed that the dominant crystal phase formed was a struvite (MgNH4PO4.6H2O) for all samples, and secondary phase Magnesium Phosphate Hydrate (Mg3(PO4)2*8H2O was found in a stirring speed of 100 rpm at 30°C. The morphology of the struvite crystals formed resembles irregular flakes by using SEM.
References
[1] A. Nabayi, C. T. B. Sung, A. T. K. Zuan, T. N. Paing, and N. I. M. Akhir, “Chemical and microbial characterization of washed rice water waste to assess its potential as plant fertilizer and for increasing soil health,” Agronomy, vol. 11, no. 12, p. 2391, 2021.
[2] I. Arliyani, B. V. Tangahu, and S. Mangkoedihardjo, “Selection of Plants for Constructed Wetlands Based on Climate and Area in the Interest of Processing Pollutant Parameters on Leachate: A Review,” in IOP Conference Series: Earth and Environmental Science, 2021, vol. 835, p. 012003.
[3] Z. Han et al., “Recovery of phosphate, magnesium and ammonium from eutrophic water by struvite biomineralization through free and immobilized Bacillus cereus MRR2,” Journal of Cleaner Production, vol. 320, p. 128796, Oct. 2021, doi: 10.1016/j.jclepro.2021.128796.
[4] H. S. Ayele and M. Atlabachew, “Review of characterization, factors, impacts, and solutions of Lake eutrophication: lesson for lake Tana, Ethiopia,” Environmental Science and Pollution Research, vol. 28, no. 12, pp. 14233–14252, 2021.
[5] I. P. Sari and H. R. Harahap, “PENGOLAHAN AIR BUNGAN LIMBAH LAUNDRY MENGGUNAKAN BOTTOM ASH SEBAGAI MEDIA ADSORPSI,” KINETIKA, vol. 12, no. 2, pp. 21–28, 2021.
[6] K. Annisa, “Efektivitas Pengolahan Air Limbah Bioetanol Berbasis Bioremediasi Menggunakan Azolla microphylla dan Pseudomonas aeruginosa,” PhD Thesis, UNS (Sebelas Maret University), 2021.
[7] A. Dargahi, R. Shokoohi, G. Asgari, A. Ansari, D. Nematollahi, and M. R. Samarghandi, “Moving-bed biofilm reactor combined with three-dimensional electrochemical pretreatment (MBBR–3DE) for 2, 4-D herbicide treatment: application for real wastewater, improvement of biodegradability,” RSC advances, vol. 11, no. 16, pp. 9608–9620, 2021.
[8] D. Saidulu, A. Majumder, and A. K. Gupta, “A systematic review of moving bed biofilm reactor, membrane bioreactor, and moving bed membrane bioreactor for wastewater treatment: Comparison of research trends, removal mechanisms, and performance,” Journal of Environmental Chemical Engineering, vol. 9, no. 5, p. 106112, 2021.
[9] T. A. Cossu, V. Lozano, G. Spano, and G. Brundu, “Plant invasion risk in the marine protected area of Tavolara (Italy),” Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology, pp. 1–7, 2022.
[10] K. A. Felix Ribeiro, C. M. de Medeiros, and J. A. Sanchez Agudo, “How effective are the protected areas to preserve endangered plant species in a climate change scenario? The case of three Iberian endemics,” Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology, pp. 1–14, 2021.
[11] D. Ekanayake, P. Loganathan, M. A. H. Johir, J. Kandasamy, and S. Vigneswaran, “Enhanced Removal of Nutrients, Heavy Metals, and PAH from Synthetic Stormwater by Incorporating Different Adsorbents into a Filter Media,” Water, Air, & Soil Pollution, vol. 232, no. 3, pp. 1–12, 2021.
[12] M. Abdulredha et al., “Zeolite as a natural adsorbent for nitrogenous compounds being removed from water,” in IOP Conference Series: Materials Science and Engineering, 2021, vol. 1067, p. 012082.
[13] M. Shen et al., “Efficient removal of microplastics from wastewater by an electrocoagulation process,” Chemical Engineering Journal, vol. 428, p. 131161, 2022.
[14] F. E. Titchou, H. Zazou, H. Afanga, J. El Gaayda, R. A. Akbour, and M. Hamdani, “Removal of persistent organic pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process,” Groundwater for Sustainable Development, vol. 13, p. 100575, 2021.
[15] C. Gaylarde, J. A. Baptista-Neto, and E. M. da Fonseca, “Plastic microfibre pollution: how important is clothes’ laundering?,” Heliyon, vol. 7, no. 5, p. e07105, 2021.
[16] K. Kasmudin, F. Fitria, and A. Artiningsih, “The Influence of Concentration Chitosan of A Shell Snail to Lower Levels of BOD and COD on Waste Laundry,” Journal of Applied Science, Engineering, Technology, and Education, vol. 4, no. 1, pp. 9–15, 2022.
[17] L. U. Zhenya et al., “Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability,” Pedosphere, 2022.
[18] D. Crutchik and J. M. Garrido, “Kinetics of the reversible reaction of struvite crystallisation,” Chemosphere, vol. 154, pp. 567–572, 2016.
[19] M. I. H. Bhuiyan, D. S. Mavinic, and R. D. Beckie, “A solubility and thermodynamic study of struvite,” Environmental technology, vol. 28, no. 9, pp. 1015–1026, 2007.
[20] A. R. Fitriana and I. Warmadewanthi, “Penurunan kadar amonium dan fosfat pada limbah cair industri pupuk,” Jurnal Teknik ITS, vol. 5, no. 2, pp. F107–F111, 2016.
[21] D. Hou, H. Yan, J. Zhang, P. Wang, and Z. Li, “Experimental and computational investigation of magnesium phosphate cement mortar,” Construction and Building Materials, vol. 112, pp. 331–342, 2016.
[22] D. S. Perwitasari, L. Edahwati, S. Sutiyono, S. Muryanto, J. Jamari, and A. P. Bayuseno, “Phosphate recovery through struvite-family crystals precipitated in the presence of citric acid: mineralogical phase and morphology evaluation,” Environmental technology, vol. 38, no. 22, pp. 2844–2855, 2017.
[23] L. Edahwati and R. R. Anggriawan, “Pembentukan Pupuk Struvite dari Limbah Cair Industri Tempe dengan Proses Aerasi,” Jurnal Teknologi Lingkungan, vol. 22, no. 2, pp. 215–221, 2021.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








