Anion Effect and Ligand Preference in the Precipitation of Ni(II) Complex from Methanolic Solution: Case of Tartrate vs Pyrazine
Abstract
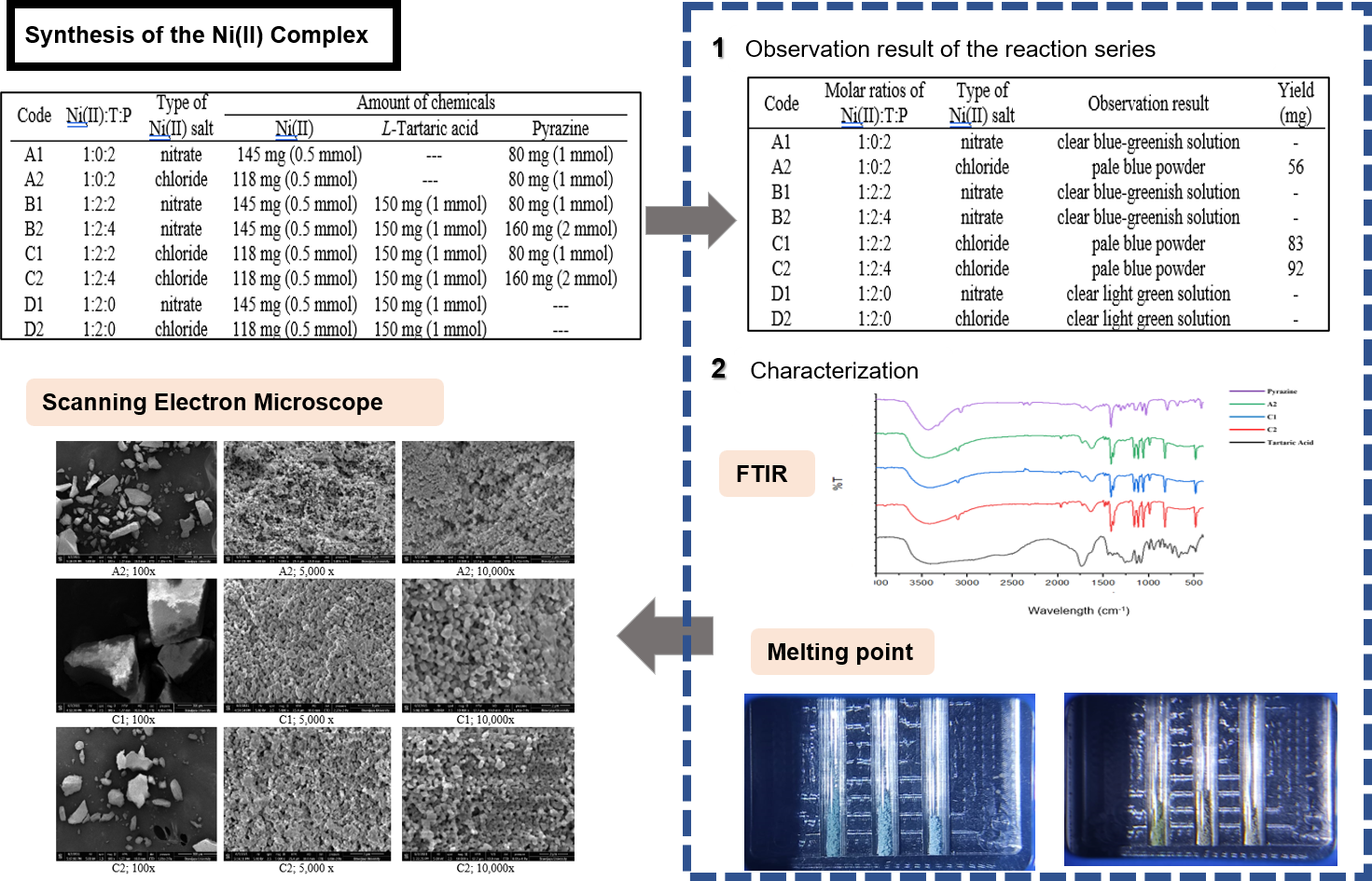
This research aims to incorporate pyrazine in the synthesis of Ni(II)-tartrate-pyrazine metal-organic frameworks or Ni(II)-T-P MOF as candidates of porous material. Synthesis of the targeted MOF was conducted at room temperature in a methanolic solution by mixing Ni(II) solution, L-tartaric acid (T), and pyrazine (P) solutions sequentially in various molar ratios (Ni(II):T:P = 1:1:0; 1:0:2; 1:2:2; and 1:2:4) using two different Ni(II) salts (chloride and nitrate). Solid products were characterized by infrared spectroscopy, qualitative anion identification test, melting point test, and scanning electron microscopy. The result shows that type of nickel salt affects the precipitation of Ni(II) complex, in which pale blue solids were precipitated out only from the chloride reactions (1:0:2, 1:2:2, and 1:2:4). IR and SEM analyses from the 1:2:2 and 1:2:4 reactions show the identical result as also shown by the 1:0:2 reaction, whereas qualitative anion identification test result suggests that the chloride is uncoordinated to the Ni(II). The targeted Ni(II)-T-P is unsuccessfully obtained, instead, the product is proposed to be [Ni(pyrazine)x(H2O)(6-x)]Cl2. although the tartaric acid was doubled and firstly reacted with the Ni(II), the pyrazine still has a higher preference to coordinate to the Ni(II) center than the tartrate ligand.
References
[1] Gu, Y and Yang, M, Cryst. Res. Technol, 2008, 43 (12), 1331–1334.
[2] Khunur, M. M and Prananto, Y.P, Bull. Chem. React. Eng. Catal, 2018, 13 (2), 213–219.
[3] Rashidipour, M., Derikvand, Z., Shokrollahi, A., Mohammadpour, Z and Azadbakht, A., Arab. J. Chem., 2017, 10, S3167–S3175.
[4] da Silva, G. B., Menezes, P. H., Malvestiti, I., FalcŃo, E. H., Alves Jr, S., Chojnacki, J and Da Silva, F. F., J. Mol. Struct., 2018, 1155, 530–535.
[5] Khunur, M. M and Prananto, Y.P., The Proceedings Book of the 8th Annual Basic Science International Conference, Malang-Indonesia, 2018, 229 – 233.
[6] Scherb, S., Nather, C and Bensch, W., Acta Crystallogr. C. Cryst. Struct. Commun., 2002, 58 (2), m135–m136.
[7] Gao, Q., Xie, Y. B and Wang, D, J. Chem. Crystallogr., 2008, 38 (8), 587–590.
[8] Palǒić, A., Puškarić, A., Mazaj, M., Žunkoviǒ, E., Logar, N. Z and Bronić, J., J. Solid State Chem., 2015, 225, 59–64.
[9] Lu, J., Liu, H. T., Wang, D. Q., Niu, M. J and Wang, S. N., J. Chem. Crystallogr., 2011, 41 (5), 641–648.
[10] Tabatabaee, M., Gholamighavamabad, A., Khabiri, E and Parvez, M., J. Inorg. Organomet. Polym. Mater., 2011, 21 (3), 627–633.
[11] Vera-Cruz, P., Toscano, R. A., Balmaseda, J., Basterrechea, M., Niño, N and del Castillo, L. F., Cryst. Eng. Comm., 2012,14 (24), 8606–8614.
[12] Feng, G. D and Jiang, L., Asian J. Chem., 2013, 25 (11), 6270.
[13] Coronado, E., Galan-Mascaros, J. R., Gomez-Garcia, C. J. and Murcia-Martinez, A., Chem. Eur. J., 2006, 12 (13), 3484–3492.
[14] Wang, Y., Liu, G. X., Chen, Y. C. Wang, K. B and Meng, S. G., Inorg. Chim. Acta, 2010, 363 (11), 2668–2672.
[15] Yan, P., Xing, J., Li, G., Sun, W., Zhang, J and Hou, G., J. Coord. Chem., 2009, 62 (13), 2095–2107.
[16] Tarigi, S and Abbasi, A., J. Nanostruct., 2012, 2, 279–288.
[17] Liu, J., Goddard, P. A., Singleton, J., Brambleby, J., Foronda, F., Möller, J. S and Manson, J. L., Inorg. Chem., 2016, 55 (7), 3515–3529.
[18] Gholipour-Ranjbar, H., Soleimani, M and Naderi, H. R., New J. Chem., 2016, 40 (11), 9187–9193.
[19] Tchalala, M. R., Bhatt, P. M., Chappanda, K. N., Tavares, S. R., Adil, K., Belmabkhout, Y and Eddaoudi, M., Nat. Commun., 2019, 10 (1), 1-10.
[20] Fang, W. X., Ma, S. H., Dong, H., Jin, X. W., Zou, Y. C., Xu, K. X and Luo, Y. H., ACS Appl. Nano Mater., 2021, 4 (5), 5541–5547.
[21] Payehghadr, M and Hashemi, S. E., J. Incl. Phenom. Macrocycl. Chem., 2017, 89 (3), 253–271.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








