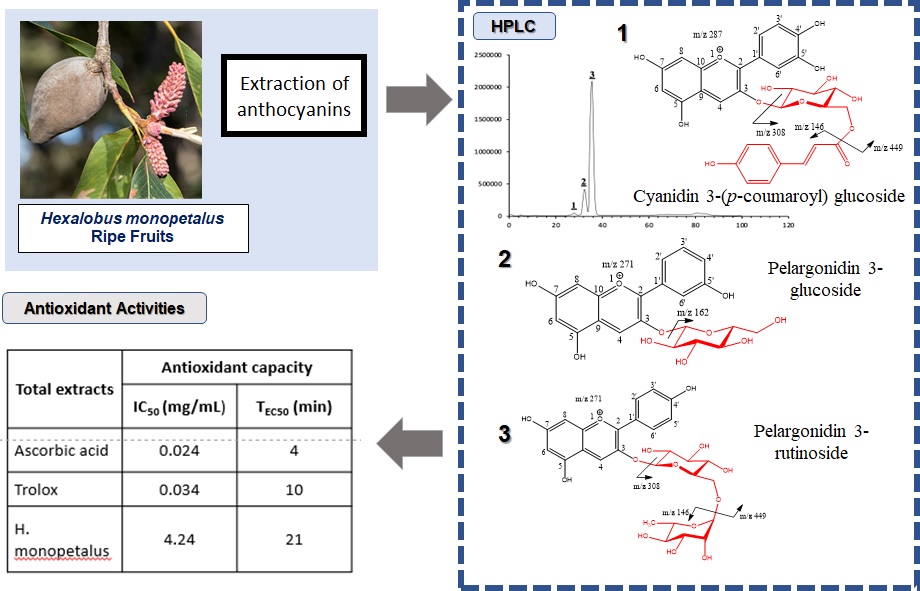
Determination of the radical-scavenging activities and identification of anthocyanins from Hexalobus monopetalus ripe fruits
Abstract
References
[1] Kouebou, C., Goygoy, F., Bourou, S., Djakissam, P.K., Layla, H., Zenabou, G., Barbi, M., Vunyingah, M and Woin, N. JABs, J. Appl. Biosci, 2013, 69, 5523–5533.
[2] Djaha, A. J and Gnahoua, G. M, JABs, J. Appl. Biosci, 2014, 78 (1), 6620–6629.
[3] Gordon, M. H, Nat. Prod. Rep, 1996, 13 (4), 265–273.
[4] Halliwell, B, Annu. Rev. Nutr, 1996, 16 (1), 33–50.
[5] Feskanich, D., Ziegler, R. G., Michaud, D. S., Giovannucci, E. L., Speizer, F. E., Willett, W. C and Colditz, G. A, J. Natl. Cancer Inst, 2000, 92 (22), 1812–1823.
[6] Lim, Y. Y., Lim, T. T and Tee, J. J, Food Chem, 2007, 103 (3), 1003–1008.
[7] Rocco, A., Fanali, C., Dugo, L., Mondello, L. Electrophoresis 2014, 35 (11), 1701–1708.
[8] Williamson, G, Nutr. Bull, 2017, 42 (3), 226–235.
[9] Schantz, M., Mohn, C., Baum, M., Richling, E, J. Berry Res, 2010, 1 (1), 25–33.
[10] Weisel, T., Baum, M., Eisenbrand, G., Dietrich, H., Will, F., Stockis, J. P., Kulling, S., Rüfer, C., Johannes, C and Janzowski, C. Biotechnol. J, 2006, 1 (4), 388–397.
[11] Habanova, M., Saraiva, J. A., Haban, M., Schwarzova, M., Chlebo, P., Predna, L., Gažo, J and Wyka, J. Nutr. Res, 2016, 36 (12), 1415–1422.
[12] Bakuradze, T., Tausend, A., Galan, J., Anna, I., Groh, M., Berry, D., Tur, J. A., Marko, D., Richling, E., Bakuradze, T., Tausend, A., Galan, J., Anna, I., Groh, M., Berry, D., Tur, J. A., Marko, D and Richling, E. Free Radic, Res. 2019, 53 (S1), 1045–1055.
[13] Singleton, V. L., Orthofer, R and Lamuela-Raventos, R. M. Methods Enzym, 1999, 299, 152–178.
[14] Zhishen, J., Mengcheng, T and Jianming, W. Food Chem, 1999, 64, 555–559.
[15] Dewanto, V., Wu, X., Adom, K. K and Liu, R. H. Journal Agric. Food Chem, 2002, 50, 3010–3014.
[16] Sakanaka, S., Tachibana, Y and Okada, Y. Food Chem, 2005, 89, 569–575.
[17] Khan, R. A, Chem. Cent, J, 2012, 6, 1–7.
[18] Wrolstad, R. E, Food Anal. Chem, 2001, 1–13.
[19] Giusti, M. M., Polit, M. F., Ayvaz, H., Tay, D and Manrique, I. J. Agric. Food Chem, 2015, 1–14.
[20] El, E., Youssef, M., Kharrassi, E., Moustaid, K., Khalid, A and Boubker, E. J. Food Meas. Charact, 2019, 13, 121–130.
[21] Fuleki, T and Francis, F. J. J. Food Sci, 1968, 33, 78–83.
[22] Popovici, C., Saykova, I and Tylkowski, B. Rev. génie Ind, 2009, 4, 26–39.
[23] Ribereau-Gayon, P. DUNOD Paris 6ème édition, 1968, 144–172.
[24] Giusti, M. M., Rodríguez-Saona, L. E., Griffin, D and Wrolstad, R. E. J. Agric. Food Chem, 1999, 47 (11), 4657–4664.
[25] Lopes-Da-Silva, F., De Pascual-Teresa, S., Rivas-Gonzalo, J and Santos-Buelga, C. Eur. Food Res. Technol, 2002, 214 (3), 248–253.
[26] Wu, X., Prior, R. L. J. Agric. Food Chem, 2005, 53 (8), 3101–3113.
[27] Horbowicz, M., Kosson, R., Grzesiuk, A., Debski, H. Veg. Crop. Res. Bull, 2008, 68, 5–22.
[28] Palé, E. Contribution à l’étude des composés anthocyaniques des plantes : cas de Hibiscus sabdariffa, Lannea microcarpa, Vigna subterranea et Sorghum caudatum du Burkina Faso., Université Joseph KI-ZERBO, Burkina Faso, 1998.
[29] Kubola, J., Siriamornpun, S., and Meeso, N. Food Chem, 2011, 126, 972–981.
[30] Lamien-Meda, A., Lamien, C. E., Compaoré, M. Y. M., Meda, N. T. R., Kiendrebeogo, M., Zeba, B., Millogo, J. F and Nacoulma, O. G. Molecules, 2008, 13, 581–594.
[31] Trappey III, R. J., Woodside, A. G. J. Advert. Res, 2005, 45 (04), 382–401.
[32] Noba, A., Koala, M., Hema, A., Bationo, R. K., Constantin, M., Palé, E., Nacro, M. African J. Pure Appl. Chem, 2020, 14 (3), 60–68.
[33] Lako, J., Trenerry, V. C., Wahlqvist, M., Wattanapenpaiboon, N., Sotheeswaran, S., Premier, R, Food Chem, 2007, 101, 1727–1741.
[34] Wang, H., Cao, G., Prior, R. L, J. Agric. Food Chem, 1997, 45 (2), 304–309.
[35] Bors, W., Heller, W., Michel, C and Saran, M, methods Enzymol, 1990, 186, 343–355.
[36] Swamy, M. K, Springer Nat. Singapore Pte Ltd, 2020, 1–592.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.