Coffee Biomass Encapsulated in Calcium Alginate as Material for Lead (II) Adsorption from Aqueous Solutions
Abstract
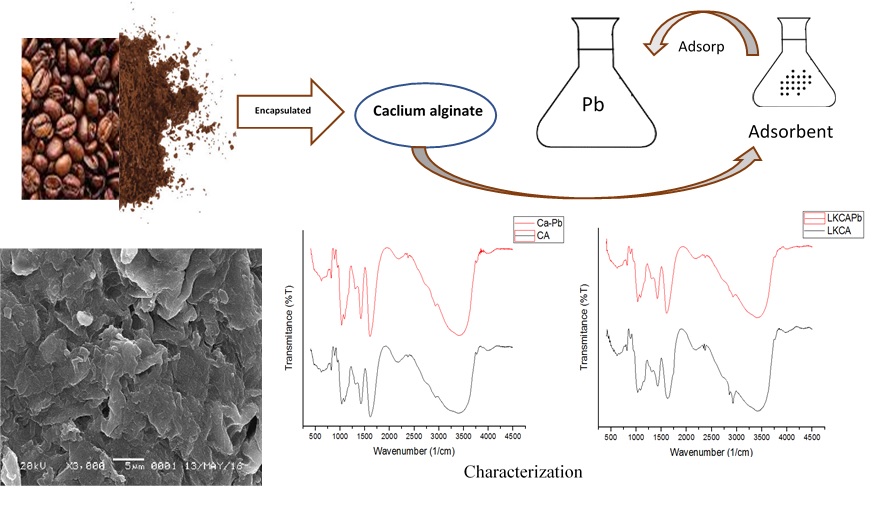
This work aims to study the removal of lead (II) from aqueous solutions with Ca-Alginate (CA) and Coffee-Calcium-Alginate (CCA). The coffee biomass were successfully prepared as the material to be encapsulated in calcium alginate. The characterization of the synthesized CA and CCA was performed using fourier transform infrared and scanning electron microscopy. The method used was batch study. Various factors which affected adsorption efficiency of lead (II) ions by CA and CCA, such as pH, agitation time, and adsorbent dose were investigated for determination of optimum experimental conditions. The result showed that CA and CCA had significant effects on adsorption of lead (ІІ) ions at pH = 4, agitation time of more than 120 min, and the adsorbent dose was 0.05 gram. Moreover, the Langmuir isotherm model showed that maximum adsorption capacity (qm) was 163.66 mg/g and 176.99 mg/g respectively for CA and CCA. The Langmuir isotherm was better described the adsorption equilibrium. Both of the adsorbent fitted to pseudo second order equations. These results demonstrated that CA and CCA show great potential to remove Pb(ІІ) ions from aqueous solutions.
References
[1] Peraturan Menteri Kesehatan Republik Indonesia Nomor 492/MENKES/PER/IV/2010 tentang Persyaratan Kualitas Air Minum.
[2] Srinivasa Rao, K., Dash, P.K., Sarangi, D., Roy Chaudhury, G., and Misra, V.N., J. Chem. Technol. Biotechnol, 2005, 80(8), 892–898.
[3] IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 87, 1–471.
[4] Gupta, S.S., and Bhattacharyya, K.G., J. Hazard. Mater, 2006, 128(2–3), 247–257.
[5] Gong, R., Ding, Y., Li, M., Yang, C., Liu, H., and Sun, Y., 2005, 64(3), 187–192.
[6] Luo, F., Liu, Y., Li, X., Xuan, Z., and Ma, J., Chemosphere, 2006, 64(7), 1122–1127.
[7] Direktorat Jenderal Perkebunan, Statistik Perkebunan Indonesia Komoditas Kopi 2014-2016, Kementerian Pertanian, Indonesia, 2015.
[8] Asosiasi Eksportir Kopi Indonesia, Konsumsi Kopi Indonesia, http://www.aeki-aice.org/tabel_konsumsi_kopi_indonesia_aeki.html, 27 May 2016.
[9] Kuczajowska-Zadrozna, M., Klimiuk, E., and Wojnowska-Baryla, I., 2004, 13(2), 161–169.
[10] Clarke, R.J., and Vitzthum, O.G., COFFEE: recent developments, 2001, Blackwell Science Ltd, Oxford.
[11] Tzu, T. W. J. Environ. Prot. (Irvine,. Calif),. 2013, 04(01), 51–55.
[12] Chen, H., Zhao, Y., and Wang, A., J. Hazard. Mater., 2007, 149(2), 346–354.
[13] Wu, D., Zhao, J., Zhang, L., Wu, Q., and Yang, Y., Hydrometallurgy, 2010, 101(1–2), 76–83.
[14] Chen, J.H., Ni, J.C., Liu, Q.L., and Li, S.X., Desaination, 2012, 285, 54–61.
[15] Hassan, A.F., Abdel-Mohsen, A.M., Elhadidy, H., Int. J. Biol. Macromol, 2014, 68, 125–130.
[16] Boparai, H.K., Joseph, M., and O’Carroll, D.M., J. Hazard. Mater, 2011, 186(1), 458–465.
[17] Amarasinghe, B.M.W.P.K., and Williams, R.A., Chem. Eng. J, 2007, 132, 299–309.
[18] Alhogbi, B.G., Sustain. Chem. Pharm, 2017, 6, 21–25.
[19] Surchi, K.M.S., 2011, Int. J. Chem, 3(3), 103–112.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








