Synthesis, Characterization, and Photocatalytic Activity of N-Doped TiO2/Zeolite-NaY for Methylene Blue Removal
Abstract
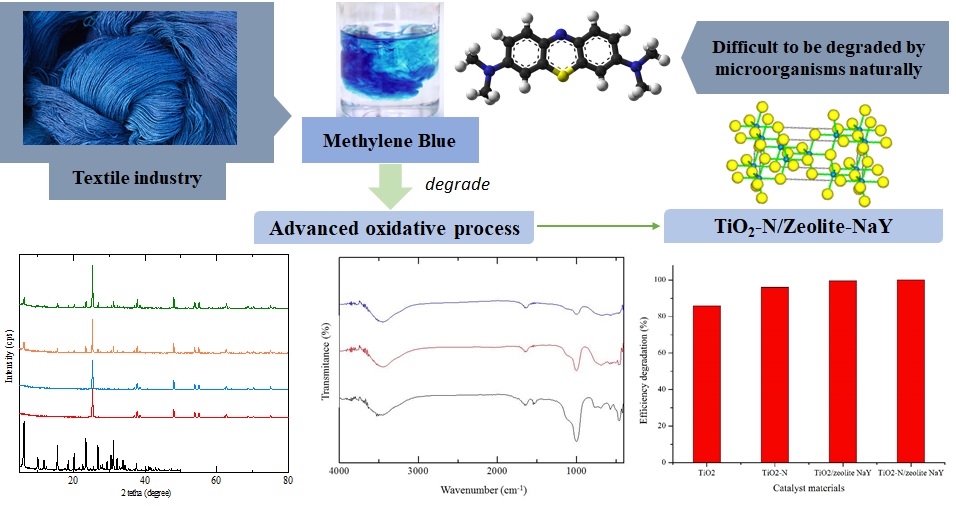
Dye is an important compound in the textile industry. The famous dye for coloring of textile is methylene blue. Methylene blue degradation has been difficult when carried out naturally by microorganisms. The advanced oxidative process is a promising method to degrade methylene blue using semiconductor material TiO2 and its modification. The modification catalyst of TiO2 such as TiO2-N, TiO2/zeolite-NaY, and TiO2-N/zeolite-NaY. These materials were synthesized by mixing TiO2 and urea, then followed by impregnation of the mixture to zeolite-NaY as support material. The materials have been synthesized then characterized by XRD, and FTIR. Degradation of methylene blue on the synthesized materials was tested under UV light for 5, 20, 30, 40, and 50 minutes. The results showed that the diffractogram of TiO-N/zeolite-NaY and TiO2/zeolite-Y has a similar specific peak with TiO2 and zeolite-NaY. It indicates that the impregnation process was successful. TiO2/zeolite-NaY and TiO2-N/zeolite-NaY also showed excellent activity for degrading methylene blue, which reached up to 99% for 3 hours of reaction.
References
[1] Damayanti, C.A., Wardhani, S. and Purwonugroho, D, Jurnal Ilmu Kimia Universitas Brawijaya, 2014, 1 (1), 8.
[2] Hidayat, W, Teknologi Pengolahan Air Limbah, 2008, Majari Magazine, Jakarta.
[3] Konwar, R.J. and De, M, Int. J. Energy Res, 2015, 39 (2), 223-233.
[4] Fansuri, H., Iryani, A., Shahbihi, W.E., Santoso, E., Hartanto, D. and Iqbal, R.M, MJFAS, 2017, 13 (4), 817-20.
[5] Susanti, I, Science Education and Application Journal, 2019, 1 (1), 10-16.
[6] Endang, P.S., Rahadian, A.R., Ulva, T.I.M., Alvin, R.W., Rendy, M.I. and Nurul, W, Materials Science Forum, 2019, 964, 199-208, Trans Tech Publications Ltd.
[7] Kalantari, K., Kalbasi, M., Sohrabi, M. and Royaee, S.J, Ceram, 2016, 42 (13), 14834-14842.
[8] Ansari, S.A., Khan, M.M., Ansari, M.O. and Cho, M.H, New J Chem, 2016, 40 (4), 3000-3009.
[9] Susanti, I. and Widiastuti, N., MJFAS, 2019, 15 (2), 240-253.
[10] Bao, N., Niu, J.J., Li, Y., Wu, G.L. and Yu, X.H, Environ. Technol, 2013, 34 (21), 2939-2949.
[11] Sakthivel, S., Janczarek, M. and Kisch, H, J. Phys. Chem. B, 2004, 108 (50), 19384-19387.
[12] Li, H., Hao, Y., Lu, H., Liang, L., Wang, Y., Qiu, J., Shi, X., Wang, Y. and Yao, J, Appl. Surf. Sci, 2015, 344, 112-118.
[13] Yang, G., Jiang, Z., Shi, H., Xiao, T. and Yan, Z, J. Mater. Chem, 2010, 20 (25), 5301-5309.
[14] Wang, H., Gao, X., Duan, G., Yang, X. and Liu, X, J. Environ. Chem. Eng, 2015, 3 (2), 603-608.
[15] Utubira, Y., Wijaya, K., Triyono, T. and Sugiharto, E, Indones. J. Chem, 2006, 6 (3), 231-237.
[16] Zarrabi, M., Entezari, M.H. and Goharshadi, E.K, Rsc Advances, 2015, 5 (44), 34652-34662.
[17] Khalil, M., Iqbal, R.M., Kadja, G.T. and Djuhana, D., Indones. J. Chem., 2020, 3 (3), 117-117.
Refbacks

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








