The Use of Low Ammonia Concentration in the Radiochemical Purity Test of [153Sm]Sm-EDTMP by Using the Thin Layer Chromatography Method
Abstract
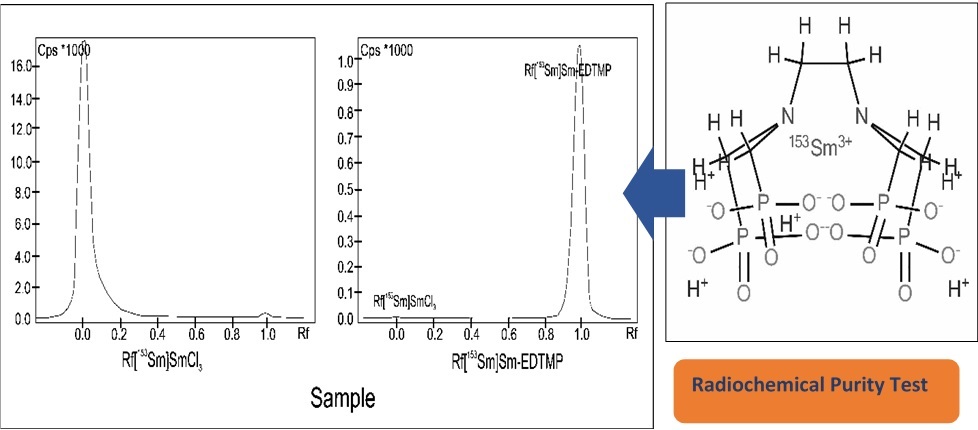
Radiochemical purity testing of [153Sm]Sm-EDTMP usually uses the Thin Layer Chromatography method. The mobile phase used is a mixture of 25% ammonia and water. However, the lowest ratio of 25% ammonia in the mobile phase is unknown. Therefore, research related to the use of the lowest concentration in the radiochemical purity test of [153Sm]Sm-EDTMP is necessary. This research method includes labelling of EDTMP using Samarium-153, preparation of the mobile phase with variations in the concentration of 25% ammonia: water, radiochemical purity test and data analysis using t-test statistics. The results of this study are the concentration of 25% ammonia: water (1: 9) to (1: 200) still shows good separation with Rf of [153Sm]SmCl3 and [153Sm]Sm-EDTMP at 0.0, 1.0 respectively, whereas with a thinner concentration of ammonia indicates less optimal separation with Rf [153Sm]SmCl3 at 0.35 to 1.0. Comparison of concentrated ammonia concentrations of 1: 9 and dilute 1: 200 was performed using a statistical t-test. The results of the data analysis showed that the two methods were not significantly different, indicated by the t-value of 0.82 less than 2.78. The conclusion of this study is that the lowest concentration of 25% ammonia and water in the radiochemical purity test of [153Sm]Sm-EDTMP is 1: 200.
References
[1] Coenen, H.H., Gee, A.D., Adam, M., Antoni, G., Cutler, C.S., Fujibayashi, Y., Jeong, J.M., Mach, R.H., Mindt, T.L., Pike, V.W., and Windhorst, A.D., Nucl. Med. Biol., 2017, 55, v–xi.
[2] U.S. Pharmacopoeia 29th edition online version, United States, Monographs, 2006, http://ftp.uspbpep.com/v29240/usp29nf24s0_m74396.html.
[3] Anderson, P.M., Subbiah, V., and Rohren, E. Current Advances in Osteosarcoma, 2014, 804, 291–304.
[4] Vigna, L., Matheoud, R., Ridone, S., Arginelli, D., Della Monica, P., Rudoni, M., Inglese, E., and Brambilla, M., Phys. Medica, 2011, 27(3), 144–152.
[5] Fallahpoor, M., Abbasi, M., Asghar Parach, A., and Kalantari, F., Appl. Radiat. Isot., 2017, 124, 1–6.
[6] Ranjbar, H., Ghannadi-Maragheh, M., Bahrami-Samani, A., and Beiki, D. Radiat. Phys. Chem, 2015, 108, 60–64.
[7] Sardari, D., and Hakimi, A. Reports Pract. Oncol. Radiother. 2012, 17(6), 358–362.
[8] Edam, A.N., Sulieman, A., Sam, A.K., Salih, I., Alkhorayef, M., and Bradley, D.A. Radiat. Phys. Chem., 2020, 167, 108247.
[9] Taylor, A.T., J. Nucl. Med., 2014, 55(4), 608–615.
[10] IAEA TECDOC SERIES – 1856, Quality Control in the Production of Radiopharmaceuticals, 2018, pp. 124–126, https://www-pub.iaea.org/MTCD/Publications/PDF/TE-1856web.pdf.
[11] Vallabhajosula, S., Killeen, R.P., and Osborne, J.R. Semin. Nucl. Med., 2010, 40(4), 220–241.
[12] Gómez-Perales, J. L., López-Martínez, E., and García-Mendoza, A. Appl. Radiat. Isot., 2016, 118, 102–104.
[13] Kadarisman, Hastini, S., Tahyan, Y., Abidin, Hafid, D., and Lestari, E. J. Radioisot. Radiopharm., 2006, 9, 13–22.
[14] International Pharmacopoeia 9th edition online version, United States,.Monographs Radiopharmaceuticals Specific monographs, 2019.https://apps.who.int/phint/en/p/docf/.
[15] Solga, S.F., Mudalel, M., Spacek, L.A., Lewicki, R., Tittel, F., Loccioni, C., Russo, A., and Risby, T.H., J. Breath Res., 2013, 7, 037101.
[16] West, C., Melin, J., Ansouri, H., and Mengue Metogo, M., J. Chromatogr. A., 2017, 1492, 136–143.
[17] Shewiyo, D.H., Kaale, E., Risha, P.G., Dejaegher, B., Smeyers-Verbeke, J., and Vander Heyden, Y., J. Chromatogr. A., 2012, 1260, 232–238.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








