Synthesis of 1-(2-Methoxybenzyl)-1,10-phenanthrolin-1-ium Bromide from Gandapura Oil
Abstract
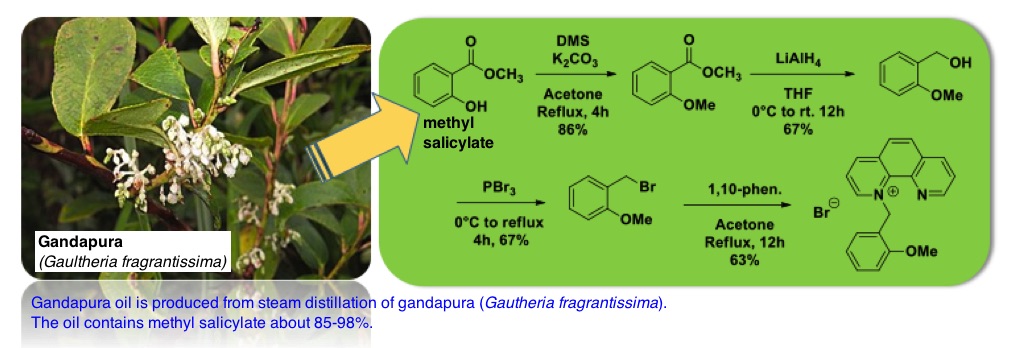
This study describes simple synthetic method to prepare 1-(2-methoxybenzyl)-1,10-phenanthrolin-1-ium bromide from gandapura oil. The salt were synthesized in four steps. Initially, commercial gandapura oil was directly subjected to the alkylation reaction under basic condition using dimethyl sulfate to give methyl 2-methxybenzoate in 86% yield. Next, the produced benzoate ester was reduced by LiAlH4 to produce 2-methoxybenzyl alcohol in 67% yield. The treatment of benzyl alcohol with phosphorus tribromide under solvent free condition produced the corresponding benzyl bromide (in 67% yield), which was directly introduced into bimolecular nucleophilic substitution reaction with 1,10-phenantroline monohydrate to finally give the desired product in 63% yield.
References
[1]. Antakli, S., Sarkis, N., Kabaweh, M., Asian J. Chem., 2010, 22 (6), 4931–4938.
[2]. Bencini, A. and Lippolis, V., Coord. Chem. Rev. 2010, 254 (17–18), 2096–2180.
[3]. Aboul-Gheit, A. K., Ahmed, S. M., El-Desouki, D. S., Abdel-Azeem, S. M., El-Shahat, M. F., Eur. J. Chem. 2011, 2 (1), 104–108.
[4]. Abebe, A. and Hailemariam, T., Bioinorg. Chem. Appl., 2016, 3607924.
[5]. Yapi, A.D., Valentin, A., Chezal, J.M., Chavignon, O., Chaillot, B., Gerhardt, R., Teulade, J.C., Blache, Y., Arch. Pharm., 2006, 339 (4), 201–206.
[6]. Yapi, A. D., Mustofa, M., Valentin, A., Chavignon, O., Teulade, J.-C., Mallie, M., Chapat, J.-P., Blache, Y., Chem. Pharm. Bull., 2000, 48 (12), 1886–1889.
[7]. Firdaus, M., Jumina, J., & Anwar, C., Indones. J. Chem., 2008, 8 (3), 423–425.
[8]. Hadanu, R., Matsjeh, S., Jumina, J., Mustofa, M., Widjayanti, M. A., & Solikhah, E. N., Indones. J. Chem. 2010, 7 (2), 197–201.
[9]. Hadanu, R., Matsjeh, S., Jumina, J., Mustofa, M., Solikhah, E. N., Widjayanti, M. A., Indones. J. Chem., 2012, 12 (2), 152–162.
[10]. Fitriastuti, D., Mardjan, M. I. D., Jumina, J., & Mustofa, M., Indones. J. Chem., 2014, 14 (1), 1–6.
[11]. Hadanu, R., Anwar, C., Jumina, J., Tahir, I., & Mustofa, M., Indones. J. Chem. 2014, 4 (2), 82–97.
[12]. Hadanu, R., Mustofa, M. & Nazudin, N., MAKARA J. Sci., 2012, 16(2), 101-109.
[13]. Wijayanti, M.A., Sholikhah, E.N., Hadanu, R., Jumina, J., Supargiyono, S., & Mustofa, M., Malar. Res. Treat., 2010, 540786.
[14]. Widjayanti, M. A., Solikhah, E. N., Tahir, I., Hadanu, R., Jumina, J., Supargiyono, S. & Mustofa, M., J. Health Sci., 2006, 52 (6), 794–799.
[15]. Solikhah, E. N., Supargiyono, S., Jumina, J., Widjayanti, M. A., Tahir, I., Hadanu, R. & Mustofa, M., Southeast Asian J. Trop. Med. Public Health, 2006, 37(6), 1072–1077.
[16]. Li, H. and Yan, C.G., Tetrahedron, 2011, 67(16), 2863–2869.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








