Simple and Rapid Device for Mercury Detection Based on The Formation of Mercury(II)-Dithizonate on Polytetrafluoroethylene (PTFE) Membrane
Abstract
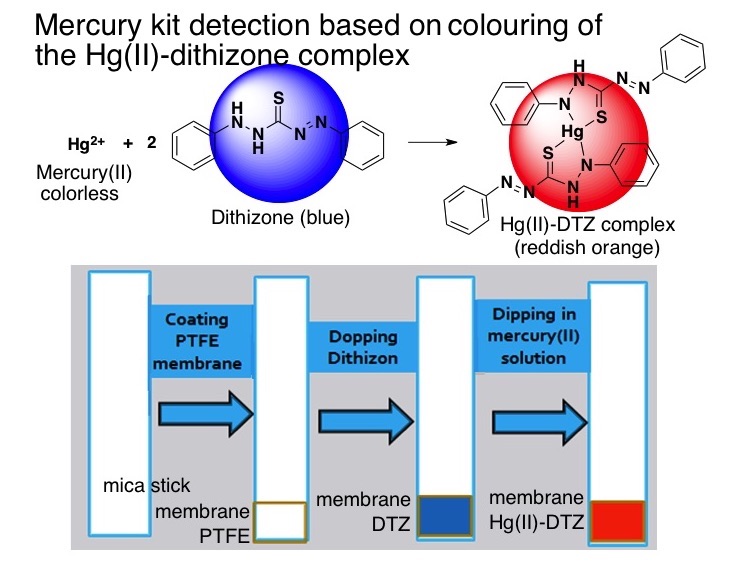
A new analytical device for mercury detection has been developed by doping dithizone on to hydrophobic PTFE (polytetrafluoroethylene) membrane to form a blue dithizone membrane which instantaneously changed to orange color of mercury(II)-dithizonate complex, when this dithizone membrane was contacted to mercury(II) solution. The higher concentration of mercury showed the greater intensity of the orange colour mercury(II)-dithizonate complex. The design and chemicals were optimized to obtain the best performance for mercury measurement. This method is prospective as mercury test kit for simple, low cost, and rapid semi-quantitative method for mercury(II) determination from 1-10 mgL-1 suits for on-site mercury detection and has been applied to cosmetics with satisfactory results.
References
[1] Park, J.D. and Zheng, W., J. Prev. Med. Public Health., 2012, 45(6), 344-352.
[2] Sah, R.C., Poisonous Cosmetics: The problem of mercury in skin whitening cream in Nepal, CEPHED, Nepal, 2012.
[3] Eaton, A.D., Clesceri, L.S., Rice, E.W. & Greenberg, A.E., American Public Health Association (APHA) Standard Method for the Examination of Water and Wastewater, 17th ed., Water Pollution Control Federation, Washington, D.C., 2005
[4] Nixon, D.E., Burrit, M.F., & Moyer, T.P., Spectrochim. Acta B., 1999, 54(8), 1141–1153.
[5] Schmit, J.P., Youla, M. and Gelinas, Y., Anal. Chim. Acta, 1991, 249(2), 495–501.
[6] Safavi, A. And Baezzat, M.R., Anal. Lett., 1996, 29(5), 807-819.
[7] Andac, M., Asan, A., Bekdemir, Y., Kutuk, H., & Isildak, I., Talanta, 2003, 60(1), 191-197.
[8] Peng, X.J., Mao, Q.K., and Cheng, J.K., Microchim. Acta, 1994, 113(1-2), 81-89.
[9] Al-Saidi, H.M., El-Shahawi, M.S. and Al-Arique, Q.H., Int. J. Appl. Chem., 2010, 6(2), 263–278.
[10] Veeranna, V., Prasad, A.R.G., Rao, V.S., An. Univ. Bucuresti Chimie, 2011, 20(1), 57-64.
[11] Danwittayakul, S., Takahasi, Y., Suzuki, T., & Thanaboonsombut, A., J. Met. Mater. Min., 2008, 18(2), 37-40.
[12] Chen, W., Fang, X., Li, H., Cao, H., & Kong, J., Sci. Rep., 2016, 6, 31948.
[13] Cai, L., Fang, Y., Mo, Y., Huang, Y., Xu, C., Zhang, Z., and Wang, M., AIP Adv., 2017, 7 (8), 085214.
[14] Sulistyarti, H., and Kolev, S.D., Microchem. J., 2013, 111, 103–107.
[15] Sulistyarti, H., Kolev, S.D., and Lim, S., Indones. J. Chem., 2010, 10(2), 167-171.
[16] Sulistyarti, H., Cardwell, T.J., and Kolev, S.D., Anal. Chim. Acta, 1997, 357(1-2), 103-109.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








