Modification of Activated Carbon from Coconut Shell Charcoal with Copper (CuCl2/AC, Cu(OH)2/AC, CuO/AC) for Adsorption of Paracetamol Contaminant
Abstract
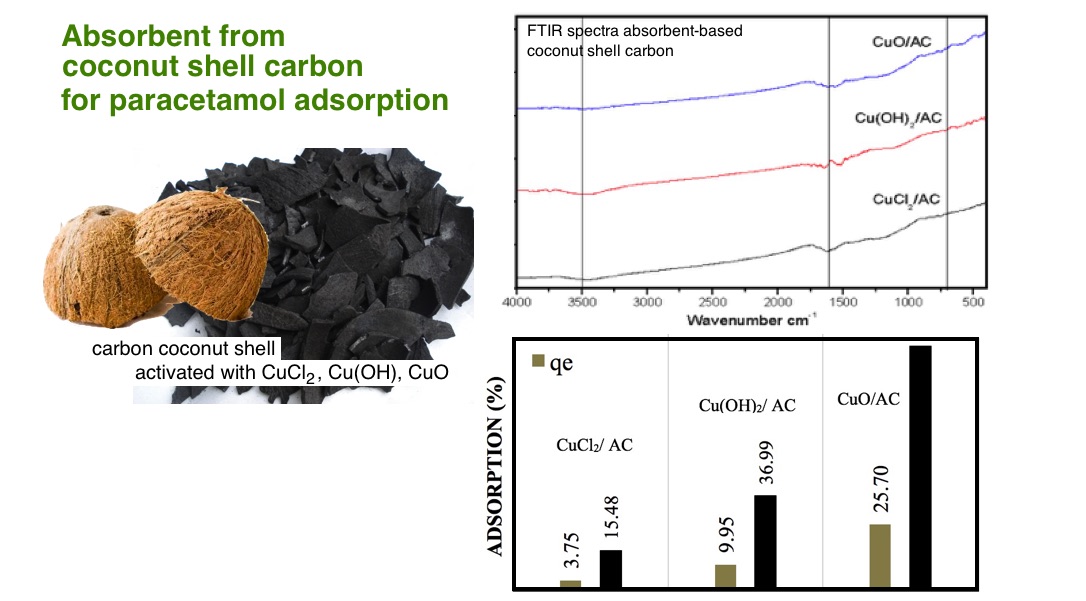
This study provides information about the physicochemical properties and performance of activated carbon combined with copper to remove paracetamol from waste models. The activated carbon (AC) comes from coconut shell charcoal. CuCl2 was used as the copper source which then combined with activated carbon (AC). The AC was obtained by activating the coconut shell charcoal using KOH and 500°C calcination for 10 minutes. Carbon functionalization were done using H2SO4 6M as an oxidizer and temperature of 80°C for 3 hours. The impregnation of activated carbon with CuCl2 produces CuCl2/AC, then the CuCl2/AC was reacted with NaOH 5M to form precipitation of Cu(OH)2/AC. CuO/AC composite was finally produced by calcining the Cu(OH)2/AC to 950°C for 5 minute. The composite was characterized by FTIR, SEM-EDX, XRF and X-ray diffraction. The adsorption of paracetamol with CuO/AC composite gave the best results of 95.56% efficiency.
References
[1] Escher, B.I., Baumgartner, R., Koller, M., Treyer, K., Lienert, J., McArdell, C.S., Water Res., 2011, 45(1), 75-92.
[2] Oktem, Y.A., Ince, O., Sallis, P., Donnelly, T. and Ince, B. K., Bioresour. Technol., 2008, 99(5),1089-1096.
[3] Deegan, A.M., Shaik, B., Nolan, K., Urell, K., Oelgemoeller, M., Tobin, J., Morrissey, A., Int. J. Environ. Sci. Technol., 2011, 8(3), 649-666.
[4] Pandey, P. and Saini, V. K., Int. J. OHSFE-Allied Sci. 2015, 5(1), 017-019.
[5] Hirasaki, G. J., J. Adhes. Sci. Technol., 1993, 7(3), 285-322.
[6] Tripathi, A. and Ranjan, M. R., J. Bioremed. Biodeg., 2015, 6(6), 1-5.
[7] Dutta, M., Das, U., Mondal, S., Bhattachriya, S., Khatun, R. and Bagal, R., Int. J. Environ. Sci., 2015, 6(2), 270.
[8] Setianingsih, T., Masruri, M., and Ismuyanto, B., Int. J. ChemTech Res., 2016, 9(12), 610-621.
[9] Liou, R. M., and Chen, S. H., J. Hazard. Mater., 2009, 172(1), 498-506.
[10] Hongo, T., Iemura, T. and Yamazaki, A., J. Ceram. Soc. Japan, 2008, 116(1350), 192-197.
[11] Meilia, D., Khunur, M. M., and Setianingsih, T., in IOP Conf. Series: Materials Science and Engineering, 2017, 299(1), 012036.
[12] Wan, J., Xuehui. J., Hui, L. and Kezheng, C., J. Mater. Chem., 2012, 22(27), 13500-13505.
[13] Parida, K.M. and Mohapatra, L., Chem. Eng. J., 2012, 179, 131-139.
[14] Park, S.J., Jang, Y.S., Shim, J.W., and Ryu, S.K., J. Colloid Interf. Sci., 2003, 260(2), 259–264.
[15] Shen, W., Li, Z., and Liu, Y., Recent Pat. Chem. Eng., 2008, 1(1), 27-40.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.