The Bioavailability and Risk Potential of Copper and Zinc of Soil as An Indicator Heavy Metals Contamination in The Aquatic System in Sumber Nyolo, Karangploso, East Java
Abstract
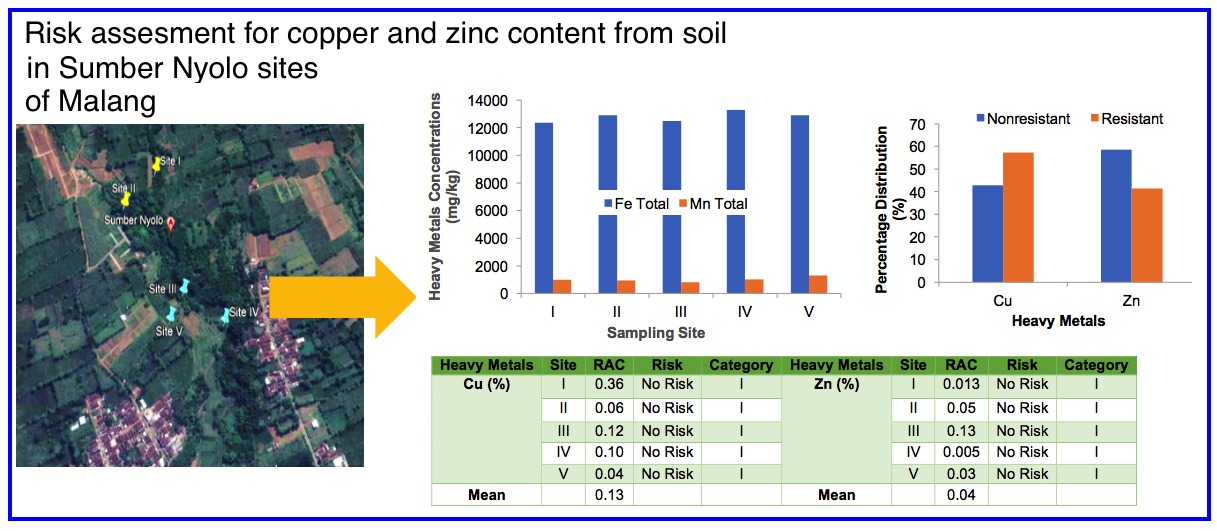
The bioavailability potential of heavy metals in the soil can be used as an indicator of heavy metals pollution in an aquatic system. This research were aimed to determine the distribution of geochemical fractions of copper and zinc in the soil, and to determine the potential risk of soil to the contamination of copper and zinc in Sumber Nyolo aquatic system. Soil samples were collected from five sites at the upper and downstream of the Sumber Nyolo by using grab sampling. Samples were pretreated before metals analysis. Geochemical fractions of copper and zinc was extracted using the Community Bereau of Reference (BCR) modified technique and followed by copper and zinc analysis using Atomic Absorption Spectroscopy. The results showed that copper was found dominantly as oxidisable fraction (F3) at 19.11 ± 11.8 mg/kg (25 ± 15.5%), while zinc was found as oxidisable fraction (F3) at 228.4 ± 283.5 mg/kg (34 ± 34.7%). As the first fraction (F1) that is referred as the bioavailable fraction, copper (13 ± 12.7%) was more potentially released from soil to water body than zinc (4 ± 5.1%), so copper was more bioavailable. Based on correlation with physical-chemical properties of soil, copper will be released when there is increasing of soil redox potential, increasing of cation exchange capacity and changes of pH to acid condition. Furthermore, the Risk Assessment Code (RAC) value for copper and zinc in soil from Sumber Nyolo were 0.13% and 0.04%, respectively. This indicated that soil in Sumber Nyolo has no harmful for copper and zinc contamination in the aquatic system. In conclusion, by understanding the distribution of geochemical fractions of copper and zinc in soil and soil potential risk, it can be predicted the potentially contamination of copper and zinc in the aquatic system which is interacted with the soil.
References
Cottenie, A. & Verloo, M., Journal of Analytical Chemistry, 1984, 317, 389–393. [2] Ratuzny, T., Gong, Z., & Wilke, B-M., Environmental Monitoring and Assessment, 2009, 156, 171–180. [3] Buhani & Suharso, Indo. J. Chem, 2006, 1, 43–46. [4] Handayanto, E., Muddarisna, N., & Fiqri, A., Pengelolaan Kesuburan Tanah, First Edition, 2017, Universitas Brawijaya Press, Malang. [5] Najamuddin, Pratono, T., Sanusi, H. S., & Nurjaya, I. W, J. Tropical Mar. Scie. and Tech., 2016, 8, 11–28. [6] Liu, Z., Pan, S., Sun, Z., Ma, R., Chen, L., Wang, Y., & Wang, S., Marine Pollution Bulletin, 2015, 98, 115–129. [7] Nemati, K., Abu Bakar, N. K., Abas, M. R., & Sobhanzadeh, E., Journal of Hazardous Materials, 2011, 192, 402–410. [8] Singh, K. P., Mohan, D., Singh, V. K., & Malik, A., Journal of Hydrology, 2005, 312, 14–27. [9] Chakraborty, P., D. Ramteke & S. Chakraborty, Marine Pollution Bulletin, 2015, 93, 194–201. [10] Li, H., Qian, X., Hu, W., Wang, Y. & Gao, H, Science of The Total Environment, 2013, 456–457, 212–221. [11] Wali, A., Colinet, G., & Ksibi, M., Environmental Research, Engineering and Management, 2014, 4(70), 14–26. [12] Honglei, L., Liqing, L., Chengqing, Y., & Baoqing, S., Journal of Environmental Sciences, 2008, 20, 390–397. [13] Sundaray, S. K., Nayak, B. B., Lin, S., & Bhatta, D., Journal of Hazardous Materials, 2011, 186, 1837–1846. [14] Levy, D. B., Barbarick., K. A., Siemer, E. G., & Sommers L. E., J. Environ. Qual., 1992, 21, 185–195. [15] Cao, J., Lam, K. C., Dawson, R. W., Liu, W. X., & Tao, S., Chemosphere, 2004, 54, 507–514. [16] Filgueiras, A. V., Lavilla, I., & Bendicho, C., Journal Environ. Monit., 2002, 4, 823–857. [17] Sutherland, R. A., Tack, F. M. G., Tolosa, C. A., & Verloo, M. G., J. Environ. Qual, 2000, 29, 1431–1439. [18] Zimmerman, A. J & David C. Weindorf, International Journal of Analytical Chemistry, 2010, 1–7. [19] Sarkar, S. K., Favas, P. J.C., Rakshit D., & Satpathy, K. K., Clarivate Anaytical Index, 2014, 723–757. [20] El-Brady, A. E. A., & El–Kammar, A. M., Marine Pollution Bulletin, 2017, 1–6. [21] Okoro, H. K., Fatoki, O. S., Adeloka, F. A., Ximba, B. J., & Snyman, R. G., Open Access Scientific Reports, 2012, 1(3), 1–9. [22] Kyziol, J., Polish Journal of Environmental Studies, 2002, 11(6), 713–718. [23] Feng, X. D., Dang, Z., Huang, W. L., & Yang, C., Journal Environ. Scie. Tech., 2009, 6(3), 337–346. [24] Delgado, J., Barba-Brioso C., Nieto J. M., & Boski, T., Science of the Total Environment, 2011, 409, 3666–3679. [25] Phillips, S. L., & Perry, D. L., Handbook of Inorganic Compounds, 1995, CRC Press.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








