Density Functional Theory (DFT) Study on α,α-Bis(2-benzothiophen-1-yl)-4H-cyclopenta[2,1-b,3;4-b′]dithiophene Derivatives for Optoelectronic Devices
Abstract
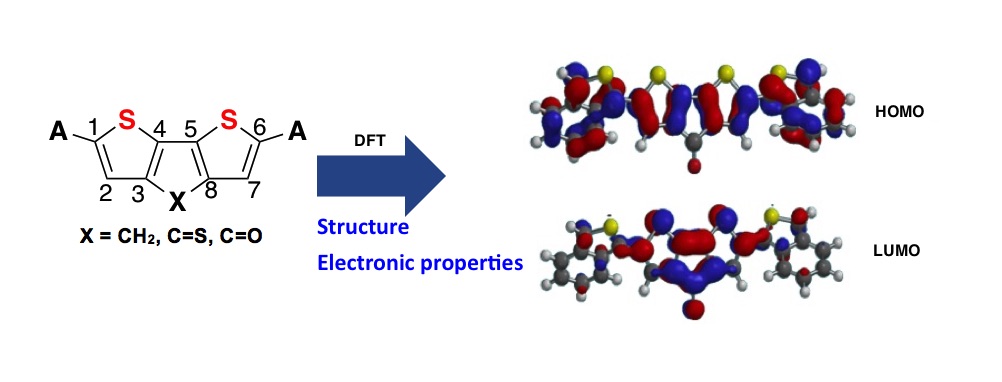
Bis(2-benzothiophen-1-yl)-4H-cyclopenta[2,1-b,3;4-b′]dithiophene derivatives comprised of three series; bis(2-thienyl)-4H-cyclopenta[2,1-b,3;4-b]dithiopene (BTDT), diphenyl-4Hcyclopenta[2,1-b,3;4-b]dithiophene (DPDT) and bis(2-benzothiophen-1-yl)-4H-cyclopenta[2,1-b,3;4-b]dithiophene (BBDT) have been studied using Density Functional Theory (B3LYP/6-31G**). In each series, molecules with C=S bridge exhibited the lowest band gap; for instance in BBDT series, the energy band gap could be arranged as 2.29, 2.23 and 1.66 eV for CH2, C=O and C=S bridge respectively. The low band gaps calculated for BBDT-C=S (1.66 eV) and BTDT-C=S (1.82 eV) could facilitate photo-excited electron transfer as one the criteria for a molecule to be used in photovoltaic devices. Also, the results showed that longest UV-vis absorption wavelength was observed for molecules with C=S bridge, i.e. 1013.66, 874.75 and 1097.66 nm for BTDT, DPDT and BBDT respectively. The polarizability (α0) valves calculated for the molecules follow as -CH2 < C=O < C=S bridge in each series, indicating that the higher the polarizability (α0) valve the longer the λmax nm and the lower the energy band gap. The magnitude of the molecular hyperpolarizability β0 showed that molecular structures with -C=O bridge could be best NLO material in each series.
References
References
[1] G. Tourillon, Polythiophene and its derivatives, in: T.A. Skotheim (Ed.), Handbook of Conducting Polymers, vol. 1 ( Dekke, Inc., New York, 1986)
[2] P. Baeuerle, Oligothiophenes in electronic materials, in: G. Wegner, K. Muellen (Eds.), The Oligomeric Approach, (Wiley-VCH, Weinheim, 1998).
[3] P. de Silva, H.Q.N. Gunaratne, T. Gunnlaugsson, A.J.M. Huxely, C.P. McCoy, J.T. Rademacher, T.E. Rice, Chem. Rev. 97, 1515 (1997)
[4] C.E. Kerr, C.D. Mitchell, J. Headrick, B.E. Eaton, T.L. Netzel, J. Phys. Chem. B., 104, 1637 (2000).
[5] U. Stalmach, H. Detert, H. Meier, V. Gebhardt, D. Haarer, A. Bacher, H.-W. Schmidt, Opt. Mater. 9, 77 (1998).
[6] M. Leuze, M. Hohloch, M. Hanack, Chem. Mater. 14, 3339 (2002).
[7] R.H. Young, C.W. Tang, A.P. Marchetti, Appl. Phys. Lett., 80, 874 (2002).
[8] W. Li, Z. Wang, P. Lu, Opt. Mater., 26, 243 (2004).
[9] F. Garnier, G. Horowitz, D. Fichou, A. Yassar, Synth. Met., 81, 163 (1996).
[10] X.-C. Li, H. Cherringhaus, F. Garnier, et al., J. Am. Chem. Soc., 120, 2206 (1998).
[11] I.S. Moreira, D.W. Franco, J. Chem. Soc. Chem. Commun., 450 (1992)
[12] K. Takahashi, T. Nihira, K. Akiyama, Y. Ikegami, E. Fukuyo, J. Chem. Soc. Chem. Commun., 620 (1992).
[13] (a) O.-K. Kim, K.-S. Lee, Z. Huang, W.B. Heuer, C.S. Paik-Sung, Opt. Mater., 21, 559 (2002); (b) O-K Kim, H. Y. Woo, J.-K. Kim, W. B. Heuer, K.-S. Lee, C.-Y. Kim, Chem. Phys. Lett., 364, 432 (2002).
[14] P. Lu and G.M. Xia J. Serb. Chem. Soc. 70(2), 201 (2005).
[15] M. Guang, G.M. Xia, Q. Fang, X.G. Xui, G.B. Xui, W. Wangi, Z.Q. Liu, G.L. Song and Y.J. Wu, Chinese Chem. Lett., 14(6), 657 (2003).
[16] G.-M. Xia, P. Lu and S.-Q. Liu, Acta Chim. Slov., 52, 336 (2005)
[17] R.P. Ortiz, M.C.R. Delgado, J. Casado, V. Hernandez, O.-K. Kim, H.Y. Woo and J.T.L. Navarrete, J. Am. Chem. Soc., 126, 13363 (2004)
[18] J. Casado, H.E. Katz, V. Hernández, J.T. López Navarrete, J Phys. Chem B., 106, 2488 (2002).
[19] A.L. Nei Marc, L. Bernardo, P. Ricardo, D. S. Barbosa, Int. J. Quant. Chem., 106, 2723 (2006).
[20] Z. El Malkia, M. Bouachrineb, M. Hamidib, L. Bejjita, and M. Haddad, J. Sci. Res., 4(1), 119 (2012).
[21] M. Bouachrine, M. Hamidi, S. M. Bouzzine and H. Taoufik, J. Appl Chem. Res. 10, 29 (2009)
[22] G. A. Jarlesson, R. G. Jeconias, C. S. C. Sheila, L. Bernardo, D. N. Jordan, J. Mol. Struct THEOCHEM, 759, 87 (2006)
[23] S.M. Bouzzine, S. Bouzakraoui, M. Bouachrine and M. Hamidi, J. Mol. Struct. (THEOCHEM), 726, 271 (2005)
[24]. S.M. Bouzzine, M. Hamidi, M. Bouachrine, Orbital, 1, 203 (2009)
[25] H.A. Aouchiche, S. Djennane, A. Boucekkine, Synth. Met., 140, 127 (2004)
[26] B. Semire, O. A. Odunola and I. A. Adejoro J. Mol. Modeling, 18(6), 2755 (2012)
[27] B. Semire and O. A. Odunola, Quimica Nova, 37(5), 833 (2014).
[28] B. Semire and O. A. Odunola. Indones. J. Chem., 15 (1), 93 – 100 (2015)
[29] A.D. Becke, J. Chem. Phys. 98, 5648, (1993).
[30] C. Lee, W. Yang, R.G. Parr, Phys. Rev. B., 37, 785 (1988).
[31] Spartan 14, wavefunction, INC, Irvine CA 92612, USA
[32] D. Mühlbacher, M. Scharber, M. Morana, Z. Zhu, D. Waller, D.R. Gaudiana, C.
Brabec, Adv. Mater., 18 2884 (2006).
[33] T.T. Steckler, K.A. Abboud, M. Craps, A.G. Rinzler, J. Reynolds, Chem. Commun.
4904 (2007).
[34] P.F. Xia, X.J. Feng, J. Lu, S.W. Tsang, R. Movileanu, Y. Tao, M.S. Wong, Adv.
Mater., 20, 4810 (2008).
[35] J.B. Asbury, Y.Q. Wang Y,E. Hao, H. Ghosh, T. Lian, Res Chem Intermed, 27, 393-406 (2001).
[36] Z.Wu, B.Fan, F. Xue, C.Adachi, J. Ouyang, Energy Mater. Sol. Cells, 94, 2230 (2010)
[37] A.Gadisa, M.Svensson, M.R.Andersson, O.Inganas, Appl. Phys. Lett., 4, 1609, (2004)
[38] Y. Sun, X. Chen, L. Sun, X. Guo, W. Lu, Chem. Phys. Lett., 38, 397 (2003).
[39] C.R. Zhang, H.S. Chen, G.H. Wang, Chem. Res. Chin. U., 20, 640 (2004).
[40] Y.X. Sun, Q.L. Hao, W.X. Wei, Z.X. Yu, L.D. Lu, X. Wang, Y.S. Wang, J. Mol. Struct.: (THEOCHEM )., 904, 74 (2009)
[41] H. Nikoofard and H. Sabzyan J. Fluorine Chem., 128(6), 668 (2007).
[42] W.H. Meyer, H. Kiess, B. Binggeli, E. Meier and G. Harbeke, Synth. Met., 10(4), 255 (1985).
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.