A Validated Ultra Performance Liquid Chromatography Method for Quantification of Metformin in Human Plasma
Abstract
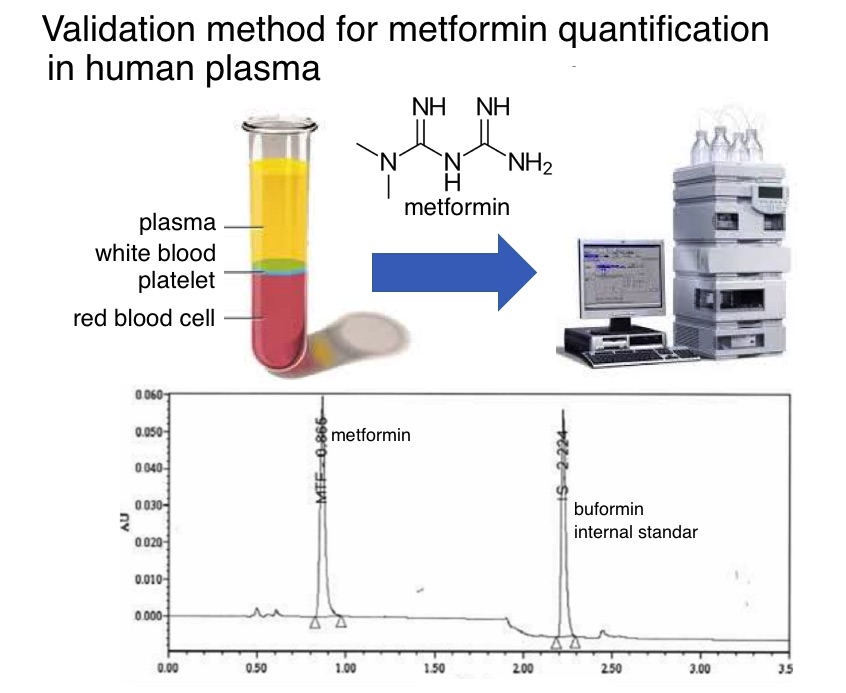
Metformin is oral hypoglycemic or blood sugar-lowering drug which is used for the first- line drug in the treatment of diabetes mellitus type 2. This study presented the validated Ultra Performance Liquid Chromatography UltraViolet (UPLC-UV) method for the determination of metformin in human plasma. Metformin levels were measured using UPLC with a UV detector and liquid-liquid extraction method. Separation was carried out on an Acquity UPLC HSS T3 100mm × 2.1mm i.d. column (1.8μm particle size) using gradient elution of acetonitrile and phosphate buffer 0.02 M (0.6 mL/min) as mobile phase at 30°C. The analyte was monitored at 236 nm. No endogenous substances were found to interfere with the peaks of drug and internal standard. The value of percent deviation and the coefficient variation obtained respectively less than the percentage set in the FDA guidelines. The linearity factor values were more than 0.997 and LOD was 0.01µg/mL. UPLC with UV detector is able to analyze metformin in a short time with good precision and accuracy which is useful for bioequivalence and bioavailability studies.
References
[1] O’Neil, M.; Heckelman, P.; Koch, C.; Roman, K. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 4th ed.; 2012, Merck & Co. , Inc: New Jersey.
[2] Nielsen, F.; Christensen, M. M. H.; Brasen, K. Quantitation of Metformin in Human Plasma and Urine by Hydrophilic Interaction Liquid Chromatography and Application to a Pharmacokinetic Study. Ther. Drug Monit. 2013, 36 (2), 211–217.
[3] Research, D. P. P.; Group. Long-Term Safety, Tolerability, and Weight Loss Associated with Metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737.
[4] American Diabetes Association. Standards of Medical Care in Diabetes - 2017. Diabetes Care 2017, 40 (Supplement 1), S33–S43.
[5] Chhetri, H. P.; Thapa, P.; Van Schepdael, A. Simple HPLC-UV Method for the Quantification of Metformin in Human Plasma with One Step Protein Precipitation. Saudi Pharm. J. 2014, 22 (5).
[6] Amini, H.; Ahmadiani, A.; Gazerani, P. Determination of Metformin in Human Plasma by High-Performance Liquid Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 824 (1–2), 319–322.
[7] Gabr, R. Q.; Padwal, R. S.; Brocks, D. R. Determination of Metformin in Human Plasma and Urine by High-Performance Liquid Chromatography Using Small Sample Volume and Conventional Octadecyl Silane Column. J. Pharm. Pharm. Sci. 2010, 13 (4), 486–494.
[8] Ranetti, M.-C.; Ionescu, M.; Hinescu, L.; Ionicǎ, E.; Anuţa, V.; Ranetti, A. E.; Stecoza, C. E.; Mircioiu, C. Validation of a HPLC Method for the Simultaneous Analysis of Metformin and Gliclazide in Human Plasma. Farmacia 2009, 57 (6), 728–735.
[9] FDA. Guidance for Industry: Bioanalytical Method Validation,; 2001, US FDA: Rockville.
[10] Ashutosh, K.; Manidipa, D.; Seshagiri, R.; Gowri, S. New Validated Stability Indicating RP-HPLC Method for Simultaneous Estimation of Metformin and Alogliptin in Human Plasma. J. Chromatogr. Sep. Tech. 2015, 6 (6), 293.
[11] Mohammed, S. A. E.; El-Dardiri, M.; Elhabak, M. A.; Abu Zaid, K. I. Determination of Metformin Hydrochloride in Human Plasma by UPLC/MS/MS: Application in Bioequivalence Study. Eur. J. Chem. 2015, 6 (2), 178–182.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








