Biosorption of Lead(II) using Trichoderma viride in the Aqueous Solution
Abstract
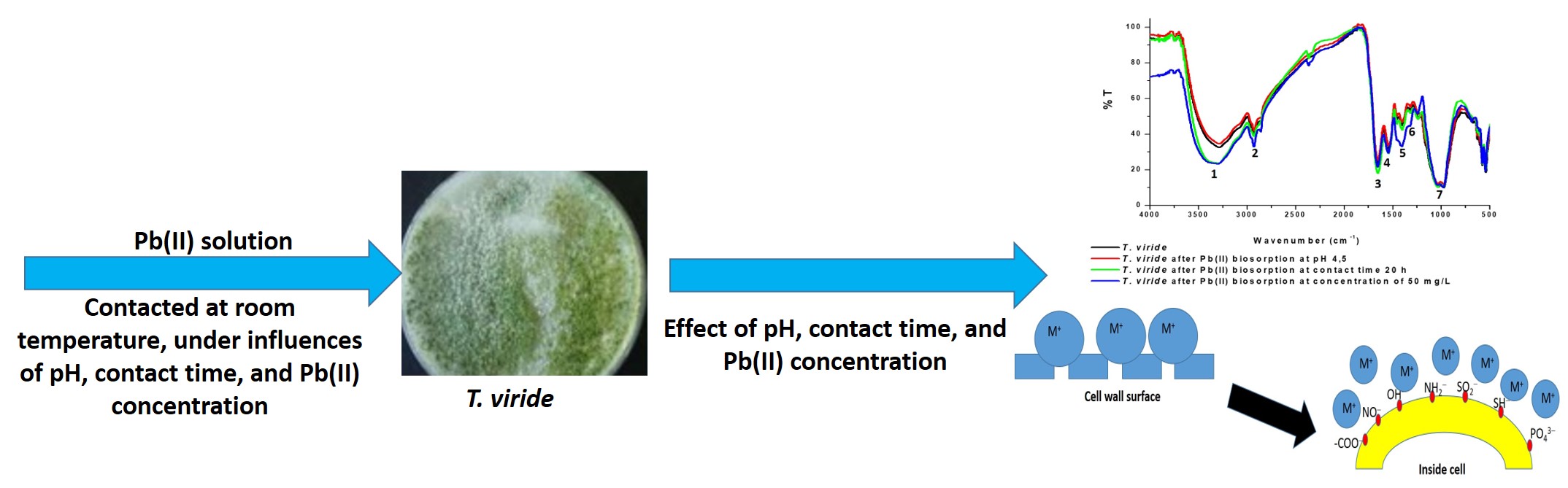
Lead(II) is considered as the main cause of pollutant that is toxic, corrosive, and irritant. One method that can be applied for reducing Pb(II) in the environment is by using microorganisms. In this work, the study of biosorption of Pb(II) in the water samples was conducted using Trichoderma viride. The research is focused on determination of optimum conditions including pH, biosorption time, and initial concentration of Pb(II) used. The profiles in functional groups contained in the T. viride have been monitored using FT-IR spectrophotometry. Results showed that the maximum biosorption of Pb(II) achieved at pH 4.5, with equilibrium of contact time of 20 h, optimum concentration of 50 mg/L, and adsorption capacity of 85 mg/1x106 T. viride colonies. The FTIR results indicated that biosorption process changed the functional groups in the T. viride. These have shown in the absorption bands at ~3200 cm-1, ~2850 cm-1, ~2260 cm-1, ~1650 cm-1, ~1450 cm-1, 1180 cm-1, and in the finger printing regions. The biosorption mechanism was proposed through the adsorption process between positively charged metal ions and the negative charge on the functional groups, such as -COO-, -OPO32-, and –NH2-, on the cell surface.
References
[1] Vazquez, C., Errasquin, E. L., Chemos., 2003, 50, 137–143.
[2] Ayangbenro, A. S., Babalola, O. O., Int. J. Environ. Res. Public Health, 2017, 14, 94–99.
[3] Ab Rahman, N. N. N., Shahadat, M., Mohd Omar, F., Chew, A. W., Omar Ab Kadir, M., Desalin. Water Treat., 2015, 2015, 1–7
[4] Mosa, K. A., Saadoun, I., Kumar, K., Helmy, M., Dankher, O. P., Front. Plant Sci., 2016, 7, 1–13.
[5] Thatoi, H., Das, S., Mishra, J., Rath, B. P., Das, N., J. Environ. Manag. 2014, 146, 383–399.
[6] Stiborova, M., Indra, R., Moserova, M., Frei, E., Schemeiser, H. H., Kopka, K., Philips, D. H., Artl, V. M., Chem. Res. Toxicol., 2016, 29, 1325–1334.
[7] Guengerich, F. P., Chem. Res. Toxicol. 2008, 21,70–83.
[8] Sujatha, P., Kalarani, V., Kumar, B. N., J. Chem. 2012, 2013, 1–7.
[9] Bishnoi, N. R., Kumar, R., Bishnoi, K., Ind. J. Biotech, 2007, 45, 657–664.
[10] Prasad, A. A., Varatharaju, G., Anushri, C., Dhivyasree, S., Br. Biotechnol. J., 2013, 3, 66–70.
[11] Abdi, A., Kazemi, M., J. Mater. Environ. Sci., 2015, 6, 1386–1399.
[12] Hemambika, B., Rani, M. J., Kannan, V. R., J. Ecol. Nat. Environ., 2011, 3, 168–175.
[13] Gadd, G. M., J. Chem. Technol. Biotechnol., 2009, 84, 13–28.
[14] Kumar, D., Gaur, J.P., Biores. Technol., 2011, 102, 2529–2535.
[15] Kumar, D., Rai, J., Gaur, J.P., Bioresour. Technol. 2012, 104, 202–207.
[16] Munro, K. L, Bambery, K. R., Carter, E. A., Puskar, L., Tobin, M. J., Wood, B. R., Dillon, C. T., Vib. Spectrosc., 2013, 53, 39–44.
[17] Barth, A., Biochim. Biophys. Acta, 2007, 1767, 1073–1101.
[18] Akhtar, K., Akhtar, M. W., Khalid, A. M., Water Res., 2007, 41, 1366–1378.
[19] Petrisor, I. G., Komnitsas, K., Lazar, I., Voicu, A., Dobrota, S., Stefanescu, M., Eur. J. Mineral Process. Environ. Protec., 2002, 2, 158–167.
[20] Igwe, J. C., Abia, A. A., African J. Biotechnol., 2006, 5, 1167–1179.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








