Melastoma malabathricum Fruit Extract-Mediated Synthesis of Silver Nanoparticles with Sensing Ability for High Concentrations of Mercury (II) Nitrate
Abstract
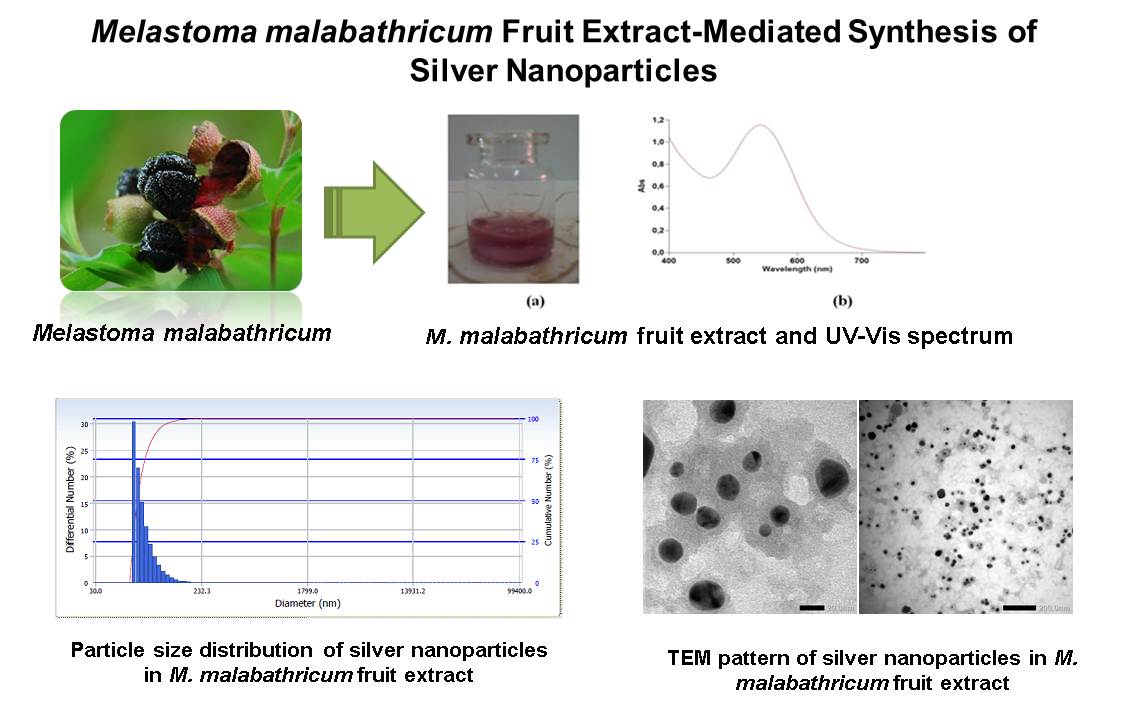
References
[1] Thakkar, K. N., Mhatre, S. S., Parikh, R.Y., Nanomed Nanotech Biol Med, 2009, 1 – 6.
[2] Bryaskova , R., Pencheva, D., Nikolov, S., Kantardijev, T., J Chem Biol., 2011, 4, 185 – 191.
[3] Suriati, G., Mariatti, M., Azizan, A., Intl. J. Automotive and Mech. Eng. (IJAME). 2014, 10, 1920 –1927.
[4] Kumar, B., Smita, K., Cumbal, L., Debut, A., Pathak, R. N., Bioinorg Chem Appl., 2014, 2014, 1-8.
[5] Wei, X., Luo, M., Li, W., Yang, L., Liang, X., Xu, L., Kong, P., Liu, H., Bioresource Tech., 2012, 103, 273-278.
[6] Lu, W., Liao, F., Luo, Y., Chang, G., Sun, X., Electrochim. Acta., 2011, 56, 2295 –2298.
[7] Zhao, X., Xia, Y., Li, Q., Ma, X., Quan, F., Geng, C., Han, Z., Colloids Surf A Physicochem Eng Asp., 2014, 444, 180-188.
[8] Philip, D., Physica E., 2010, 42, 1417 -1424.
[9] Sharma, V. K., Yngard, R. A., Lin, Y., Adv. Colloid Interface Sci., 2009, 145, 83–96,
[10] Irvani, S., Green Chem., 2011, 2638 – 2650.
[11] Ahmed, S., Ahmad, M., Swami, B. L., Ikram S., J. Adv. Res., 2016, 7: 17–28.
[12] Ahmed, M., Alsalhi, M. S., Siddiqui, M. K. J., Clin. Chim. Acta., 2010, 411, 1841-1848.
[13] Bonde, S., Nusantara Bioscie., 2011, 3, 59-63.
[14] Farhadi, K., Farough, M., Molaei, R., Hajizadeh, S., Rafipour, A., Sensor Actuat B-Chem, 2011, 1-6.
[15] Chen, L., Fu, X., Lu, W., Chen, L., ACS Appl. Mater. Interfaces, 2013, 5, 284−290.
[16] Annadhasan, M., Muthukumarasamyvel, T., Babu, V.R.S., Rajendiran, N., ACS Sustainable Chem. Eng. 2 (4), 887 – 896.
[17] Some parts of this paper was presented (oral presentation only) in Green Development International Conference organized by University of Jambi, Indonesia. October 2016.
[18] Joffry, M S. M., Yob, N. J., Rofiee, M. S., Affandi, M. M. R. M. M., Suhaili, Z., Othman, F., Akim, A. Md., Desa, M. N. M., Zakaria, Z. A., Evid. Based Complement. Alternat. Med., 2012, 2012, 1-48.
[19] Sayuti, K., Azima, F., Marisa, M., Intl. J. Advance Sci.and Eng. Information Tech., 2015, 5, 369 – 401.
[20] Yudha S. S., Suharto, T.E., Angasa, E., Nishina, Y., Mardlia, Z.A., Sipriyadi, Oriental J. chem. 2017, 33(2), 745 – 751.
[21] Makarov, V. V., Love, A. J., Sinitsyna, O. V., Makarova, S. S., Yaminska, I. V., Taliansky, M. E., Kalinina, N. O., Acta Naturae., 2014, 6, 35 – 44.
[22] During the preparation of this manuscript, we learned that similar study on the utility of silver nanoparticles for colorimetric detection of mercury. The nanoparticles was synthesized using aqueous fruits extract of water apple (Syzygium aqueum), see: Firdaus, M.L., Fitriani, I., Wyantuti, S., Hartati, Y.W., Khaydarov, R., Mchalister, J.A., Obata, H., Gamo, T., Analytical Sci. 2017, 3, 831-837.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








