Liquid Chromatography for Analysis of Metformin in Myrmeleon sp.
Abstract
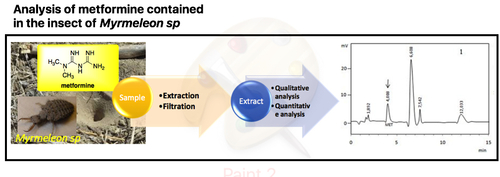
Myrmeleon sp is a typical of insect larva which has been used in Indonesia for diabetes treatment. However, there is no sufficient scientific report explaining the bioactive compounds in this insect. Based on our preliminary research, this insect contained metformin, i.e. one of bioactive compounds for the treatment of type-2 diabetes. Therefore, this study is focused on the development of separation technique using high performance liquid chromatography (HPLC) on a reverse phase C-18 column with UV detection to identify and quantify metformin in methanol extract of Myrmeleon sp. Several parameters of HPLC method were optimized with respect to high resolution of separation and accurate determination of metformin. Satisfied separation was obtained under gradient elution mode using aqueous methanol mobile phase varied from 50-90 % of methanol with flow rate of 0.5 mL/min and detection wavelength of 233 nm. The method performed total separation for all compounds in less than 11 minutes. Spiking technique was chosen for metformin identification and quantitation. Metformin in extract Myrmeleon sp was eluted at retention time (tR) of 4.095 minutes, similarly with retention time of standard metformin of 4.092 minutes. The quantity of metformin in Myrmeleon sp can be simply determined by comparing the additional area of standard metformin with area of metformin from extract Myrmeleon sp. The results confirmed that methanol extract of Myrmeleon sp contained anti-diabetes compound of metformin of 0.58 mg/g Myrmeleon sp larvae with acceptable coefficient variation of 5.56 %.
References
[1] Adler, A.I., Shaw, E.J. Stokes, T. and Ruiz, F., BMJ Journal, 2009, 338, b1668.
[2] Nathan, D.M., Buse, J.B., Davidson, M.B., Ferrannini, E., Holman, R.R., Sherwin, R., and Zinman, B., Diabetes Care, 2009, 32, 193-203.
[3] Selvin, E., Bolen, S., Yeh, H.C., Wiley, C., Wilson, L.M., Marino poulos, S.S., Feldman, L., Vassy, J., Wilson, R., Bass, E.B., and Brancati, F.L., Arch Intern Med., 2008. 168, 2070-2080.
[4] Lamanna, C., Monami, M., Marchionni, N., and Mannucci, E., Diabetes Obes Metab, 2011, 13, 221-228.
[5] Ali M. Qaisi, Maha F. Tutunji, Charl A. Sahouri., Saudi Pharm. J., 2006, 1(42), 108-118.
[6] C.-L. Cheng, C.-H. Chou, J. Chromatogr. B., 2001, 762, 51-58.
[7] Lad, N. R., Bhoir, S. I., Bhoir, I. C., and Sundaresan, M., Indian J. Pharm. Sci, 2003, 65, 650-653.
[8] Srinivas, P., Venkataramana, K., Srinivasa Rao, J., and Srinivasa, N., IJPCBS, 2012, 2, 104-109.
[9] Bhanu R., Kulkarni S., and Kadam A., Indian Drugs, 2006, 43 (1), 16-20.
[10] Wahed, M.I.I., Research Journal of Medicine and Medical Sciences, 2007, 2(2), 115-121.
[11] Florentin Tache and Monica Albu, Rev. Roum. Chim., 2007, 52 (6), 603-609.
[12] Lakshmi, M. S., Rajesh, T., and Shrinivas Sharma, Int J Pharm Pharm Sci, 2009, 1(2), 162-166.
[13] Cheng C.L., and Chou C.H., J Chromatogr B Biomed Sci Appl., 2001, 762, 51-57.
[14] Sultana, N., Naveed, S. and M. Arayne, S., J Anal Bioanal Tech 5, 2013, 4, 1-8.
[15] Peraman, R., Peruru, K.K., Yiragam, P.R., and Gowra, C.S., MJPS, 2014, 12(1), 33-46.
[16] Kurniasih, T., Isma’il, M., Susilowati, F. and Lestari, S.P., PKMP, 2006, 2 (8), 1-7.
[17] Siswandono and Soekardjo, B., Kimia Medisinal, 2008, Pusat Penerbitan dan Percetakan Unair, Surabaya.
[18] Guo-Ping, Z., Bi H.-C., S. Zhou, X. Chen, and M. Huang, J. Mass Spectrom., 2005, 40 (11), 1462-1471.
[19] C. Yardımcı, N. Özaltın, A. Gürlek, Talanta, 2007, 72, 1416-1422.
[20] U.S. Departemen of Health and Human Service, Food and Drug Administration, Guidance for Industry, Bioanalitycal Method Validation, 2001, Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM), USA.
[21] Ravichandran, V., Shalini, S., Sundram, K.M., and Rajak, H., Int J Pharm Pharm Sc, 2010, 2(3), 18-22.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








