Particle Processing of Acetaminophen using Cooling and Anti-solvent Crystallization Methods
Abstract
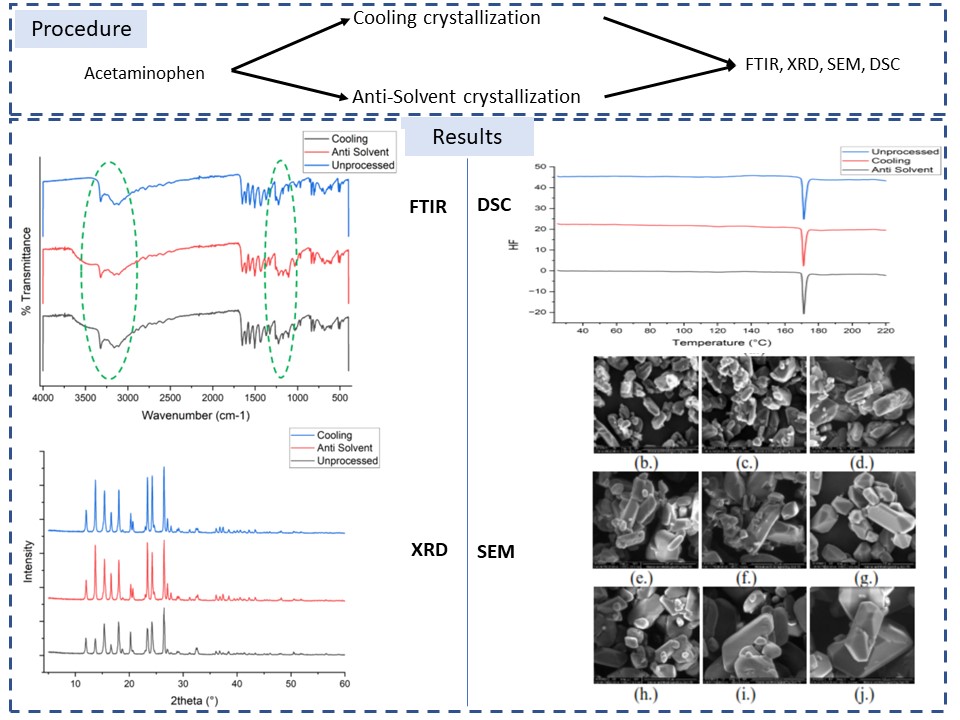
Several crystallization strategies are being implemented to enhance the solubility of acetaminophen, also known as paracetamol. Therefore, this study aimed to determine the effectiveness of the cooling and anti-solvent crystallization on the properties of acetaminophen. The results showed that the anti-solvent recrystallization produced achieved an impressive yield of up to 41.40%, while the cooling method yielded approximately 2–9%. Through FTIR, XRD, and DSC analysis on the samples, it was discovered that these methods had minimum effects on the molecular and thermal properties of the compound. Furthermore, the methods did not alter the crystallinity of acetaminophen, with crystal form I dominating the products. In addition, processing using the cooling and anti-solvent effectively reduced particle size of acetaminophen from 68.60 μm to 26.92 μm and 28.25 µm, respectively. In conclusion, the solubility of paracetamol in aqueous solutions experienced a significant enhancement. In the unprocessed samples, it measured 0.0107 gram/ml, while the recrystallized samples had an improved range of 0.0123 to 0.0167 g/ml.
References
[1] B. Okereke, O. Obeleme, A. Bin Oyemachi, Journal of International Medical Research, 2021, Vol 49 (3), pp. 1-8.
[2] A. Kustriyani and H. B. Hidayati, Journal of Pain Headache and Vertigo, 2020, Vol 1 No 2, pp. 42-47.
[3] S. Ayoub, Temperature, 2021, Vol 8 no 4, pp. 351 - 371.
[4] M.Barzegar - Jalali, P. Jafari, A. Jouyban. Journal of Molecular Liquids, 2021, Vol 349, p. 118199.
[5] K.Foe, Kelarutan Asetaminofen di Dalam Sistem Propilen Glikol - Gliserol - Air, Skripsi S1, 1989, Fakultas Farmasi Universitas Airlangga Surabaya.
[6] BPOM, Penanganan Kasus Cemaran Etilen Glikol dan Dietilen Glikol (EG/DEG) Dalam Sirop Obat, 2023, BPOM, Jakarta.
[7] R. M. Jose, R. del and W. R. Ronald, Crystal Growth and Design, 2006, vol. 6, no. 6, pp. 1407-1414.
[8] Delmas, U. Shah, M. Roberts, D. Williams, J. Heng, Powder Technology, 2013, Vol 236, pp. 24 - 29.
[9] Nandan, J. Parambil, Crystals, 2023, 13, p. 1094.
[10] S. Chewle, F. Emmerling, M. Weber, Crystals, 2020, 10, p. 1107.
[11] W, Kaialy, H. Larhrib, B. Chikwanha, S. Shojaee, A. Nokhodchi, International Journal of Pharmaceutics, 2014, 464, pp. 53 - 64.
[12] Sudha, K. Srinivasan, International Journal of ChemTech Research, 2014, Vol 6 no 3, pp. 1630 - 1632.
[13] Nishigaki, M. Maruyama, S. Tanaka, H. Yoshikawa, M. Imanishi, M. Yoshimura, Y. Mori, K. Takano, Crystals, 2021, 11. p.1069.
[14] Maeno, T. Fukami, M. Kawahata, K. Yamaguchi, T. Tagami, T. Ozeki, T. Suzuki, K. Tomono, International Journal of Pharmaceutics, 2014, 473, pp.179 - 186.
[15] Sawalha, Integrated Synthesis and Crystallization for the Continuous Manufacturing of Paracetamol, Master Thesis, 2018, TU Graz.
[16] Jiang, X. Ni, Organic Process Research and Development, 2019, 23, 5, pp. 882 - 890.
[17] A. L. Abhijit and R. P. Sanjaykumar, International Journal of Chemical Engineering and Applications, 2013, vol. 4, no. 5, pp. 337-341.
[18] S. T. Niraj, A. B. Jared, S. K. Umesh and S. T. Lynne, The Journal of Physical Chemistry, 2014, pp. 9974−9982.
[19] F. Zapata, A Lopez – Fernandez, F. Ortega – Ojeda, G Quintanilla, C. Garcia - Ruiz, G. Montalvo, Journal of Chemical Education, 2021, Vol 98, 8, pp. 2675 – 2686.
[20] Nandiyanto, A. B., Oktiani, R., & Ragadhita, R. Indonesian Journal of Science & Technology, 2019, 4, PP. 97-118.
[21] Larkin, P. J, 2011. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation. Oxford: Elsevier Inc.
[22] K. Suresh, J. Ashe and A. Matzger, Journal of Pharmaceutical Sciences, 2019, vol. 108, pp. 1915-1920.
[23] I.-C. Wang, M.-J. Lee and D.-Y. Seo, AAPS PharmSciTech, 2011, Vol. 12, No. 2, June.
[24] E. P. Firman, S. Nikmatin and R. Langenati, Jurnal Sains Materi Indonesia, 2006, pp. 154-162.
[25] EC Jones, LM Bimbo, Pharmaceutics, 2020, 12, p. 214.
[26] H Purohit, N Trasi, D Sun, E Chow, H Win, X Zhang, Y Gao, L Taylor, Journal of Pharmaceutical Sciences, 2018, Vol 107, Issues 5, pp. 1320 - 1324.
[27] J. Mullin, Crystallization Fourth Edition, London: Reed Educational and Professional Publishing Ltd, 2001.
[28] H Shekaari, MT Zafarani - Moattar, Mokhtapour, Journal of Advanced Chemical and Pharmaceutical Materials, 2018, Vol 1 Issue 2, pp. 34 - 37.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








