Syntheses, Characterization, Antioxidant Activity, and Toxicity Evaluation of Schiff Base Derivates from O-Vanillin
Abstract
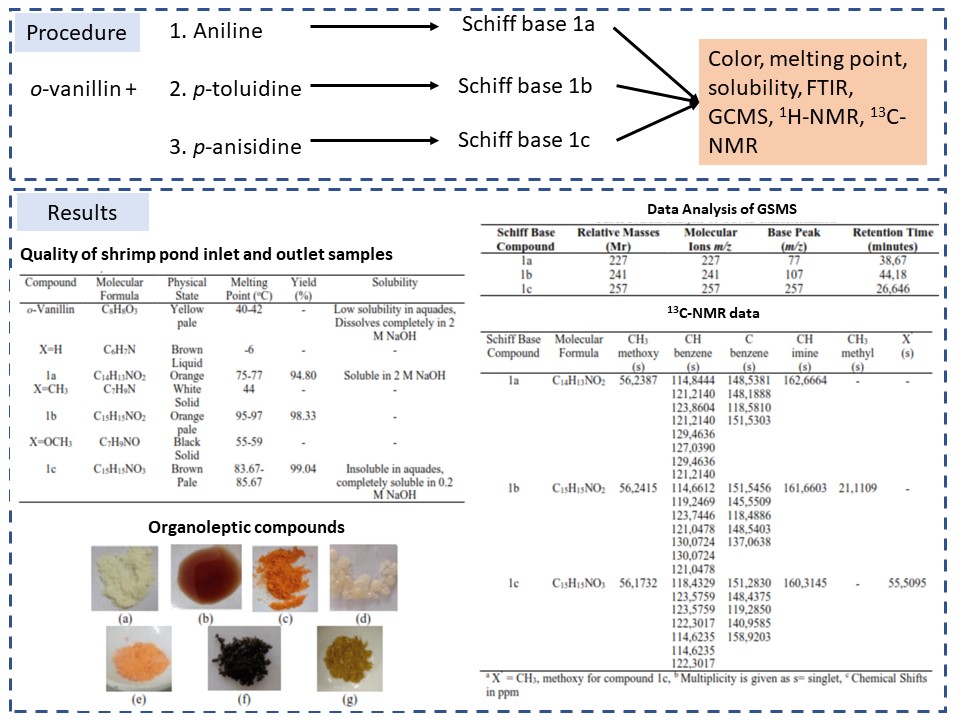
Three Schiff bases (1a, 1b, and 1c) have been prepared from the reaction of o-vanillin with primary amine (aniline, p-toluidine, and p-anisidine). Schiff base derivates from o-vanillin were synthesized using the grinding method for 20 minutes. Physical properties were observed based on color, melting point, and solubility. Synthesis products were also characterized using FTIR, GCMS, 1H-NMR, and 13C-NMR. The antioxidant activity of the Schiff base was tested using DPPH. While the toxicity test uses the BSLT method. The result of this synthesis and characterization Schiff base (1a, 1b, and 1c) showed that the Schiff base compound was formed into 2-methoxy-6 (phenyliminomethyl) phenol; 2-methoxy-6- (((4-methylphenyl) imino) methyl) phenol; and 2-methoxy-6-(((4-methoxyphenyl) imino) methyl) phenol. The result of NMR analysis, on 1H-NMR spectrum showed the shift chemical at 8,5-8,6 ppm which indicates the typical peak of proton (-HC=N-). Meanwhile, the 13C-NMR spectrum shown the shift chemical at 160-162 ppm which indicates the typical peak of carbon (-C=N-). The result of antioxidant activity showed that all Schiff base was antioxidant quite low ability with value of EC50 is 106.2-196.4 ppm. Meanwhile, the result of toxicity test showed that all Schiff base was anticancer with an LC50 value of 9.99-22.29 ppm.
References
(1) Waziri, I., Yusuf, T. L., Akintemi, E., Kelani, M. T., Muller, A. J. Mol. Struct. 2023, 1273, 134382.
(2) Poonia, K., Siddiqui, S., Arshad, M., Kumar, D. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2016, 155, 146–154.
(3) Hosny, S., Ragab, M. S., Abd El-Baki, R. F. Sci. Rep. 2023, 13 (1), 1502.
(4) Kaushik, S., Paliwal, S. K., Iyer, M. R., Patil, V. M. Med. Chem. Res. 2023, 32 (6), 1063–1076.
(5) Tople, M. S., Patel, N. B., Patel, P. P., Purohit, A. C., Ahmad, I., Patel, H. J. Mol. Struct. 2023, 1271, 134016.
(6) Apak, R., Özyürek, M., Güçlü, K., Çapanoğlu, E. J. Agric. Food Chem. 2016, 64 (5), 997–1027.
(7) Anouar, E. H., Raweh, S., Bayach, I., Taha, M., Baharudin, M. S., Di Meo, F., Hasan, M. H., Adam, A., Ismail, N. H., Weber, J.-F. F. J. Comput. Aided Mol. Des. 2013, 27, 951–964.
(8) Al Zoubi, W., Kim, M. J., Salih Al‐Hamdani, A. A., Kim, Y. G., Ko, Y. G. Appl. Organomet. Chem. 2019, 33 (11), e5210.
(9) Hassan, S. A., Aziz, D. M., Abdullah, M. N., Bhat, A. R., Dongre, R. S., Ahmed, S., Rahiman, A. K., Hadda, T. B., Berredjem, M., Jamalis, J. J. Mol. Struct. 2023, 1292, 136121.
(10) Shoaib, M., Rahman, G., Shah, S. W. A., Umar, M. N. Bangladesh J. Pharmacol. 2015, 10 (2), 332–336.
(11) McLaughlin, J. L., Rogers, L. L., Anderson, J. E. Drug Inf. J. 1998, 32 (2), 513–524.
(12) Yang, Z., Sun, P. Molbank 2006, 2006 (6), M514.
(13) Sennappan, M., Krishna, P. M., Ranganathan, R. J. Mol. Struct. 2019, 1179, 86–91.
(14) Joshi, K., Rojivadiya, A., Pandya, J. Int. J. Inorg. Chem. 2014, 2014, 817412.
(15) Hasanah, U., Hanapi, A., Ningsih, R. In Proceedings of the International Conference on Green Technology, 2017, Vol. 8, pp 278–281.
(16) Sharma, O. P., Bhat, T. K. Food Chem. 2009, 113 (4), 1202–1205.
(17) Goueti, B., Kpadonou-Kpoviessi, B., Fatondji, R., Atchade, B., Toukam, P. D., Kpoviessi, S. J. Pure Appl. Chem. Res. 2023, 12 (2), 57.
(18) Niksic, H., Becic, F., Koric, E., Gusic, I., Omeragic, E., Muratovic, S., Miladinovic, B., Duric, K. Sci. Rep. 2021, 11 (1), 13178.
(19) Meyer, B., Ferrigni, N., Putnam, J., Jacobsen, L., Nichols, D., McLaughlin, J. L. Planta Med. 1982, 45 (05), 31–34.
(20) Nisyak, K., Rahman, M. F., Sutrisno, S. J. Pure Appl. Chem. Res. 2013, 2 (1), 11–18.
(21) Silverstein, R. M., Webster, F. X., Kiemle, D. J., Bryce, D. L. Spectrometric identification of organic compounds, 8th ed., John Wiley & Sons, USA, 2015.
(22) Socrates, G. Infrared Characteristic Group Frequences. Chichester, NY, Brisbane, 3rd ed., John Wiley & Sons, England, 1994.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.