Effects of Preparation Temperature and Liquid-Solid Lipid Composition to Curcumin-Nanostructured Lipid Carrier Characteristics Fabricated by Microfluidic Technique
Abstract
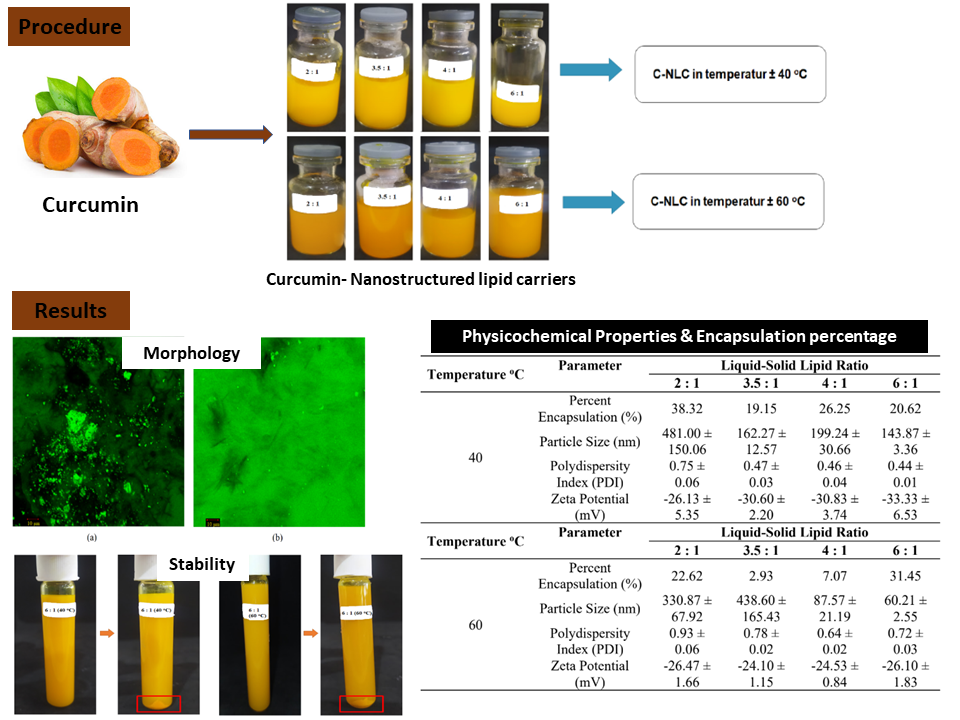
Nanostructured Lipid Carriers (NLC) are lipid-based carrier that uses a combination of liquid and solid lipids which is believed to deliver a higher amount of active substance to the human body. This study aimed to obtain the best formulation and evaluate the stability of curcumin-loaded NLC (C-NLC) using microfluidic technique at temperature of 40oC and 60oC with the ratios of liquid:solid lipids were 2 : 1 ; 3.5 : 1 ; 4 : 1 ; 6 : 1% w/w. Our results showed that the increase of process temperature and liquid lipid concentration reduced particle size. There was a non-linear relationship between lipid ratio and temperature to encapsulation percentage. At ratio of soybean oil:stearic acid 6 : 1 and, at 40°C, particles size (PS) obtained was 143.87 ± 3.36 nm, polydispersity index (PDI) obtained was 0.44 ± 0.01, zeta potential (ZP) obtained was -33.3 ± 6.53 mV with encapsulation percentage of 20.62%. At the same ratio at 60°C, the PS obtained was 60.21 ± 2.55 nm, PDI obtained was 0.72 ± 0.03, ZP obtained was -26.10 ± 1.83 mV and encapsulation percentage of 31.45%. Stability test showed that C-NLC produced at 60°C was more stable since the change of particle size and pH were lower than C-NLC produced at 40°C.
References
[1] Sangsen, Y., Laochai, P., Chotsathidchai, P., Wiwattanapatapee, R. Adv. Mater. Res. 2014, 1060, 62–65.
[2] Nastiti, C., Ponto, T., Abd, E., Grice, J., Benson, H., Roberts, M. Pharmaceutics 2017, 9 (4), 37.
[3] Khurana, S., Bedi, P. M. S., Jain, N. K. Int. J. Biomed. Nanosci. Nanotechnol. 2012, 2 (3/4), 232.
[4] Montenegro, L., Lai, F., Offerta, A., Sarpietro, M. G., Micicchè, L., Maccioni, A. M., Valenti, D., Fadda, A. M. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112.
[5] Mehanna, M. M., Mneimneh, A. T. Adv. Pharm. Bull. 2020, 11 (1), 56–67.
[6] Poonia, N., Kharb, R., Lather, V., Pandita, D. Future Sci. OA 2016, 2 (3), FSO135.
[7] Ruktanonchai, U., Bejrapha, P., Sakulkhu, U., Opanasopit, P., Bunyapraphatsara, N., Junyaprasert, V., Puttipipatkhachorn, S. AAPS PharmSciTech 2009, 10 (1), 227.
[8] Aditya, N. P., Macedo, A. S., Doktorovova, S., Souto, E. B., Kim, S., Chang, P.-S., Ko, S. LWT - Food Sci. Technol. 2014, 59 (1), 115–121.
[9] López-García, R., Ganem-Rondero, A. J. Cosmet. Dermatol. Sci. Appl. 2015, 05 (02), 62–72.
[10] Mura, P., Maestrelli, F., D’Ambrosio, M., Luceri, C., Cirri, M. Pharmaceutics 2021, 13 (4), 437.
[11] Borges, G. S. M., Prazeres, P. H. D. M., Souza, Â. M. de, Yoshida, M. I., Vilela, J. M. C., Silva, A. T. M. e, Oliveira, M. S., Gomes, D. A., Andrade, M. S., Souza-Fagundes, E. M. de, Ferreira, L. A. M. Braz. J. Pharm. Sci. 2021, 57, e18497.
[12] Erawati, T., Hariyadi, D. M., Rosita, N., Purwanti, T. Res. J. Pharm. Technol. 2021, 5719–5724.
[13] Saleem, K., Khursheed, Z., Hano, C., Anjum, I., Anjum, S. Nanomaterials 2019, 9 (12), 1749.
[14] Feng, J., Huang, M., Chai, Z., Li, C., Huang, W., Cui, L., Li, Y. Food Funct. 2020, 11 (6), 5223–5239.
[15] Gupta, A., Eral, H. B., Hatton, T. A., Doyle, P. S. Soft Matter 2016, 12 (11), 2826–2841.
[16] Sholihat, S. I., Indahyanti, E., Lestari, M. L. A. D., Ningsih, Z. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833 (1), 012044.
[17] Hamano, N., Böttger, R., Lee, S. E., Yang, Y., Kulkarni, J. A., Ip, S., Cullis, P. R., Li, S.-D. Mol. Pharm. 2019, 16 (9), 3957–3967.
[18] Puglia, C., Frasca, G., Musumeci, T., Rizza, L., Puglisi, G., Bonina, F., Chiechio, S. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2012, 81 (2), 288–293.
[19] Rapalli, V. K., Kaul, V., Waghule, T., Gorantla, S., Sharma, S., Roy, A., Dubey, S. K., Singhvi, G. Eur. J. Pharm. Sci. 2020, 152, 105438.
[20] Wang, J., Tang, J., Zhou, X., Xia, Q. Drug Dev. Ind. Pharm. 2014, 40 (2), 201–210.
[21] kaur, S., Nautyal, U., Singh, R., Singh, S., Devi, A. Asian Pac. J. Health Sci. 2015, 2 (2), 76–93.
[22] Mukherjee, S., Ray, S., Thakur, R. Indian J. Pharm. Sci. 2009, 71 (4), 349.
[23] Shah Zakaria, Mohd. R., Basri, M., Huong, C. K., Ismail, Z., Misran, M., Kassim, A., Salleh, A. B., Rahman, Mohd. B. A., Rahman, R. N. Z. R. A. J. Dispers. Sci. Technol. 2012, 33 (3), 332–338.
[24] Adhikary, T., Basak, P. In Advances in Oil-Water Separation, Das, P., Manna, S., Pandey, J. K., Eds., Elsevier, 2022, pp 511–535.
[25] Ansari, M. J., Rahman, M., Alharbi, K. S., Altowayan, W. M., Ali, A. M. A., Almalki, W. H., Barkat, Md. A., Singh, T., Nasar, S., Akhter, M. H., Beg, S., Choudhry, H. ACS Omega 2022, 7 (11), 9452–9464.
[26] Singleton, W. S., Ward, T. L., Dollear, F. G. J. Am. Oil Chem. Soc. 1950, 27 (4), 143–146.
[27] Li, M., Kang, W., Li, Z., Yang, H., Jia, R., He, Y., Kang, X., Zheng, Z., Wang, Y., Sarsenbekuly, B., Gabdullin, M. Phys. Fluids 2021, 33 (7), 072002.
[28] Ebtavanny, T., Soeratri, W., Rosita, N. Int. J. Drug Deliv. Technol. 2018, 8.
[29] Savić, V., Ilić, T., Nikolić, I., Marković, B., Čalija, B., Cekić, N., Savić, S. Int. J. Pharm. 2019, 569, 118624.
[30] Loo, Basri, M., Ismail, Lau, Tejo, B. A., Kanthimathi, M., Hassan, Choo. Int. J. Nanomedicine 2012, 13.
[31] Yeo, Y., Park, K. Arch. Pharm. Res. 2004, 27 (1), 1.
[32] Fard Masoumi, H. R., Basri, M., Sarah Samiun, W., Izadiyan, Z., Lim, C. J. Int. J. Nanomedicine 2015, 10, 6469–6476.
[33] Borrin, T. R., Georges, E. L., Moraes, I. C. F., Pinho, S. C. J. Food Eng. 2016, 169, 1–9.
[34] Ningsih, Z., Syaiful, S. P. D., Lestari, M. L. A. D., Mardiana, D., Kamulyan, B. J. Pure Appl. Chem. Res. 2023, 12 (1), 47–56.
[35] Pan, Y., Tikekar, R. V., Nitin, N. Int. J. Pharm. 2016, 511 (1), 322–330.
[36] Fang, J.-Y., Fang, C.-L., Liu, C.-H., Su, Y.-H. Eur. J. Pharm. Biopharm. 2008, 70 (2), 633–640.
[37] Sentosa, O., Katherine, Sugih, A. K. In 2015 4th International Conference on Instrumentation, Communications, Information Technology, and Biomedical Engineering (ICICI-BME), IEEE, Bandung, Indonesia, 2015, pp 332–336.
[38] Ren, W., Tian, G., Zhao, S., Yang, Y., Gao, W., Zhao, C., Zhang, H., Lian, Y., Wang, F., Du, H., Xiao, H., Zheng, J. LWT 2020, 133, 109954.
[39] Maryam Nejadmansouri, Seyed Mohammad Hashem Hosseini, Mehrdad Niakosari, Gholam Hossein Yousefi, Mohammad Taghi Golmakani. پژوهش های علوم و صنایع غذایی ایران 2017, 1396 (13).
[40] Floyd, K. A., Eberly, A. R., Hadjifrangiskou, M. In Biofilms and Implantable Medical Devices, Deng, Y., Lv, W., Eds., Woodhead Publishing, 2017, pp 47–95.
[41] In Studies in Interface Science, Hao, T., Ed., Electrorheological Fluids, Elsevier, 2005, Vol. 22, pp 235–340.
[42] Zetapotential und Partikelladung in der Laborpraxis: Einführung in die Theorie, praktische Meßdurchführung, Dateninterpretation ; Colloidal Drug Carriers - cdc - 1st Expert Meeting Berlin 15. - 17. 6 1995 ; mit 24 Tabellen, Müller, R. H., Nitzsche, R., Paulke, B.-R., Mehnert, W., Hildebrand, G. E., Eds., Paperback APV, Wiss. Verl.-Ges, Stuttgart, 1996.
[43] Wirawan, W. Indones. Food Nutr. Prog. 2023, 19 (1), 31.
[44] Obeidat, W. M., Schwabe, K., Müller, R. H., Keck, C. M. Eur. J. Pharm. Biopharm. 2010, 76 (1), 56–67.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








