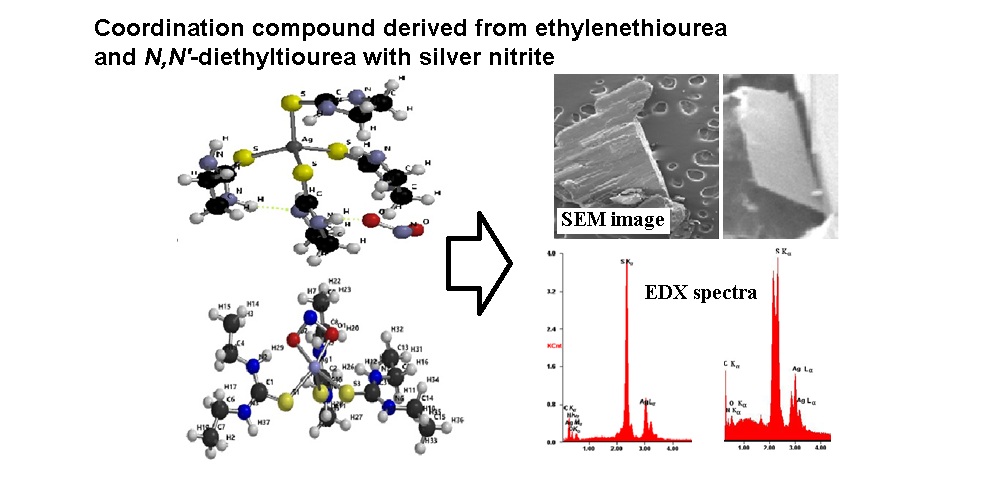
Synthesis and Characterization of Coordination Compounds of Silver(I) Nitrite with Ligands Ethylenethiourea and N,N'-diethylthiourea
Abstract
References
[1] G. A. Bowmaker, C. Pakawatchai, S. Saithong, B. W. Skelton, and A. H. White, Dalton Trans., 2009, 2588-2598
[2] G. A. Bowmaker, C. Pakawatchai, S. Saithong, B. W. Skelton, and A. H. White, Dalton Trans., 2010, 39, 4391-4404
[3] M. Altaf, H. Stoeckli-Evans, G. Murtaza, A. A. Isab, S. Ahmad, and M. A. Shaheen, J. Struct. Chem., 2011, 52(3), 625-630
[4] S. Ahmad, M. Altaf, H. Stoeckli-Evans, T. Ruffer, H. Lang, M. Mufakkar, and A. Waheed, J Chem Crystallogr, 2010, 40, 639-645
[5] S. Ahmad, Q. Amir, G. Naz, A. Fazal, M. Fettouhi, A. A. Isab, T. Ruffer, and H. Lang, J Chem Crystallogr, 2012, 42, 615-620
[6] F. B. Stocker, D. Britton, V. G. Young-Jr, Inorg. Chem., 2000, 39, 3479-3484
[7] G.A. Bowmaker, N. Chaichit, C. Pakawatchai, B.W. Skelton, and A.H. White, Can. J. Chem., 2009, 87 (1), 161-170.
[8] G. A. Bowmaker, C. Pakawatchai, B.W. Skelton, Y. Thanyasinkul, and A. H. White, Z. Anorg. Allg. Chem., 2008, 634, 2583-2588.
[9] C. Pakawatchai, S. Saithong, P. Sudkeaw, U.Tooptakong and J. H. Charmant, Analyt. Sci., 2005, 21, 211-212
[10] A. Cingolani, Effendy, M. Pellei, C. Santini, B. W. Skelton, and A. H. White, Inorg. Chem., 2002, 41, 6633-6645.
[11] N. Kornblum, H. E. Ungnade, Org. Synth., Coll, 1963, 4, 724.
[12] Pearson, R.G, J. Am. Chem. Soc., 1963, 85 (22), 3533-3539.
Refbacks
- There are currently no refbacks.









