Shortcut Approach to 1,4-Diazepine from 3-Pyridylnitrene Intermedietes under Mild Condition
Abstract
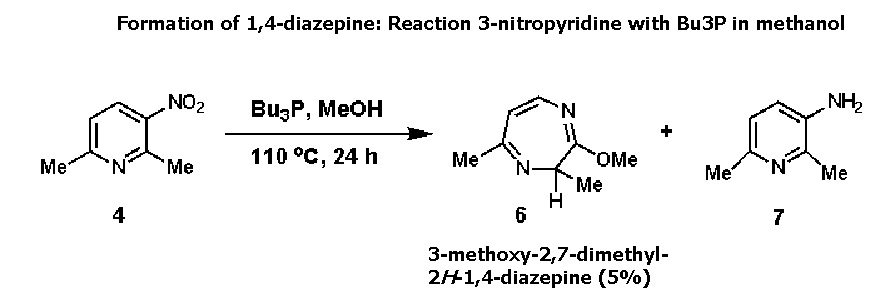
References
Hoffmann, N. Chem. Rev. 2008, 108, 1052. [2] Bach, T. and Hehn, J. P., Angew. Chem., Int. Ed. 2011, 50, 1000. [3] Atherson, F. R., and Lambert, R. W., J. Chem. Soc., Perkin Trans. 1, 1973, 1079 [4] T. de Boer, Cadogan, J. I. G., McWilliam, H. M., and Rowly, A. G., J. Chem. Soc., Perkin Trans. 2, 1975, 554. [5] Rachedine, K., Norah, B., Saliha, B., Sihame, B., Cherifa, R., and Bellara N-K., Molecules, 2011, 16, 92. [6] Dipak, P., Partha, P. B., Baikuntha, J. G., and Jagir, S. S., Beilstein J. Org. Chem., 2006, 2, 5. [7] Sawanishi, H., Tajima, K., Osada, M., and Tsuchiya, T., Chem. Pharm. Bull., 1984, 32, 4694. [8] Sawanishi, H., Tajima, K., and Tsuchiya, T., Chem. Pharm. Bull., 1987, 35, 4101. [9] Ulfa, M. S., Okamoto, H., and Satake, K., Heterocycles, 2011, 83, 1259. [10] Katritzky, A. R., Scriven, E. F. V., Majumder, S., Akhmedova, R. G., Vakulenko, A. V., Akhmedov, N. G., Murugan, R., and Abboud, K. A., Org. Biomol. Chem., 2005, 3, 538. [11] Plazek E., Chem. Ber., 1939, 72B, 577. [12] Pouchert, C. J. and J. Behnke, The Aldrich Library of 13C and 1H FT NMR Spectra, 1993. [13] Ulfa, S. M., Okamoto, H., and Satake, K., Chem. Lett., 2012, 41, 400. [14] Software employed: M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
Refbacks
- There are currently no refbacks.









