Membranes of Nata de coco-nanoparticles Fe3O4 For Diazinon Sensors
Abstract
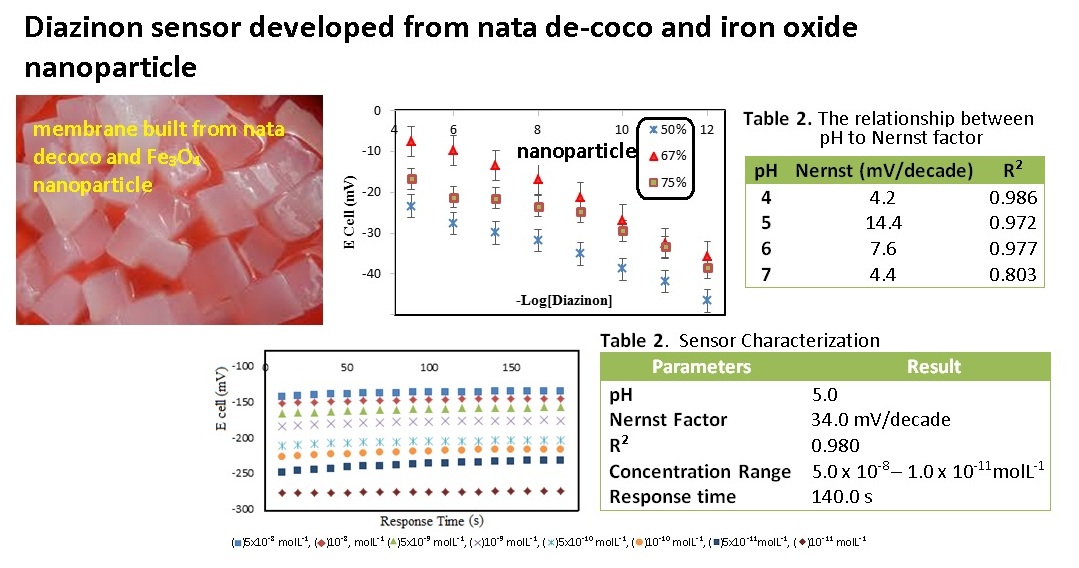
Development of diazinon sensors using mixed membranes of nata de coco and Fe3O4 nanoparticles on the SPCE (screen printed carbon electrode) surface has been carried out potentiometrically. The design of the sensor begins with the optimization of Fe3O4 nanoparticles added to the membranes of 50%, 67% and 75% while pH optimization using pH 4-5 acetate buffer and phosphate buffer pH 6-7. The diazinon sensor of the result has been tested at range concentration of 10-12 - 10-5mol.L-1. The results showed that the best sensor performance was 67% Fe3O4 nanoparticles and pH 5. The Nernst factor was 34.5 mV / decade, the detection limit value of 1,3 x 10-9mol.L-1 in the range concentration of 10-11 - 5x10-8 molL-1 with a response time of 140 seconds.
References
[1] Q. Guo et al., ‘Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery’, Int. J. Nanomedicine, p. 1697, Mar. 2012. crossref
[2] I. Nedkov, T. Merodiiska, L. Slavov, R. E. Vandenberghe, Y. Kusano, and J. Takada, ‘Surface oxidation, size and shape of nano-sized magnetite obtained by co-precipitation’, J. Magn. Magn. Mater., vol. 300, no. 2, pp. 358–367, May 2006. crossref
[3] D. Zhang et al., ‘Magnetite (Fe3O4) Core−Shell Nanowires: Synthesis and Magnetoresistance’, Nano Lett., vol. 4, no. 11, pp. 2151–2155, Nov. 2004.
[4] H. Cui, W. Yang, X. Li, H. Zhao, and Z. Yuan, ‘An electrochemical sensor based on a magnetic Fe3O4 nanoparticles and gold nanoparticles modified electrode for sensitive determination of trace amounts of arsenic(iii)’, Anal. Methods, vol. 4, no. 12, p. 4176, 2012.
[5] Y. Yang, Y. Zhang, J.-C. Shen, H. Yang, Z.-G. Zhou, and S.-P. Yang, ‘A highly selective magnetic sensor with functionalized Fe/Fe 3 O 4 nanoparticles for detection of Pb 2+’, Chin. Chem. Lett., vol. 27, no. 6, pp. 891–895, Jun. 2016.
[6] A. Kaushik, Raju Khan, Pratima R Solanki, and Pratibha Pandey, ‘Iron oxide nanoparticles–chitosan composite based glucose biosensor’, Elsevier, vol. 24, no. 4, pp. 676–683, Dec. 2008.
[7] N. Sanaeifar, M. Rabiee, M. Abdolrahim, M. Tahriri, D. Vashaee, and L. Tayebi, ‘A novel electrochemical biosensor based on Fe3O4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose’, Anal. Biochem., vol. 519, pp. 19–26, Feb. 2017.
[8] W. Zhang et al., ‘Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/graphene nanocomposites’, Sci. Rep., vol. 5, no. 1, Sep. 2015.
[9] T. Yin and W. Qin, ‘Applications of nanomaterials in potentiometric sensors’, TrAC Trends Anal. Chem., vol. 51, pp. 79–86, Nov. 2013.
[10] Ö. Yavuz, M. K. Ram, and M. Aldissi, ‘Electromagnetic applications of conducting and nanocomposite materials’, in The New Frontiers of Organic and Composite Nanotechnology, Elsevier, 2008, pp. 435–475.
[11] F. G. Nejad, H. Beitollahi, and S. Shakeri, ‘Magnetic Core–shell Fe3O4@ SiO2/Graphene Nanocomposite Modified Carbon Paste Electrode for Voltammetric Determination of Ascorbic Acid in the presence of L-Cysteine’, vol. Vol. 8, No. 3, 2016, 318-328, 2016.
[12] M. H. Mashhadizadeh and H. Jeilanpour, ‘Voltammetric Investigation of Ketotifen Using Carbon Paste Electrode Modified by Magnetic Core-Shell Fe3O4@ TMSPT Nanoparticles’, Anal Bioanal Electrochem, vol. 6, pp. 308–320, 2014.
[13] E. M. Rosida, A. Mulyasuryani, and R. T. Tjahjanto, ‘Modification of Screen Printed Carbon Electrode (SPCE) with Fe3O4 for the Determination of Nitrite (NO2-) in Squarewave Voltammetry’, Molekul, vol. 12, no. 2, p. 139, Nov. 2017.
[14] U. D. of Health and H. Services, Toxicological profile for diazinon. 2000.
[15] B. S. Nasional, ‘Batas maksimum residu pestisida pada hasil pertanian’, SNI, vol. 7313, no. 2008, pp. 70–79, 2008.
[16] S. Karanth, ‘Diazinon’, in Encyclopedia of Toxicology, Elsevier, 2014, pp. 55–56.
[17] A. Motaharian, F. Motaharian, K. Abnous, M. R. M. Hosseini, and M. Hassanzadeh-Khayyat, ‘Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples’, Anal. Bioanal. Chem., vol. 408, no. 24, pp. 6769–6779, Sep. 2016.
[18] J. Wang, M. Yokokawa, T. Satake, and H. Suzuki, ‘A micro IrOx potentiometric sensor for direct determination of organophosphate pesticides’, Sens. Actuators B Chem., vol. 220, pp. 859–863, Dec. 2015.
[19] M. Khadem et al., ‘Development of a specific electrochemical sensor for occupational and environmental monitoring of Diazinon’, Health Saf. Work, vol. 7, no. 1, pp. 9–22, 2017.
[20] Silva NAD, Birolli WG, Seleghim MHR, and Porto ALM, ‘Applied bioremediation-active and passive approaches.’, Rij. 385 InTech, 2013.
[21] D. C. Pieda, ‘Acid and Base Catalysed Aqueous Hidrylysis of the Organophosphorus Pesticide, Diazinon’, Queens Univ. Kingston Ont. Can., 2001.
[22] E. Bakker and K. Chumbimuni-Torres, ‘Modern directions for potentiometric sensors’, J. Braz. Chem. Soc., vol. 19, no. 4, pp. 621–629, 2008.
[23] Y.-J. Liang et al., ‘Size-dependent electromagnetic properties and the related simulations of Fe 3 O 4 nanoparticles made by microwave-assisted thermal decomposition’, Colloids Surf. Physicochem. Eng. Asp., vol. 530, pp. 191–199, Oct. 2017.
[24] R. R. Kalantary, Y. D. Shahamat, M. Farzadkia, A. Esrafili, and H. Asgharnia, ‘Heterogeneous photocatalytic degradation of diazinon in water using nano-TiO2: Modeling and intermediates’, Eur J Experimen Biol, vol. 4, pp. 186–94, 2014.
[25] Y. Ku, J.-L. Chang, and S.-C. Cheng, ‘Effect of solution pH on the hydrolysis and photolysis of diazinon in aqueous solution’, Water. Air. Soil Pollut., vol. 108, no. 3, pp. 445–456, 1998.
[26] P. R. Chawla, I. B. Bajaj, S. A. Survase, and R. S. Singhal, ‘Microbial cellulose: fermentative production and applications’, Food Technol. Biotechnol., vol. 47, no. 2, pp. 107–124, 2009.
[27] SNI, ‘Tanah – Bagian 1: Cara uji pestisida organoklorin secara ekstraksi menggunakan pelarut n-heksan dengan Kromatografi Gas- Spektrofotometer Massa (KG-SM)’, Badan Stand. Nas. SNI 06-69911-2004, 2008.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.