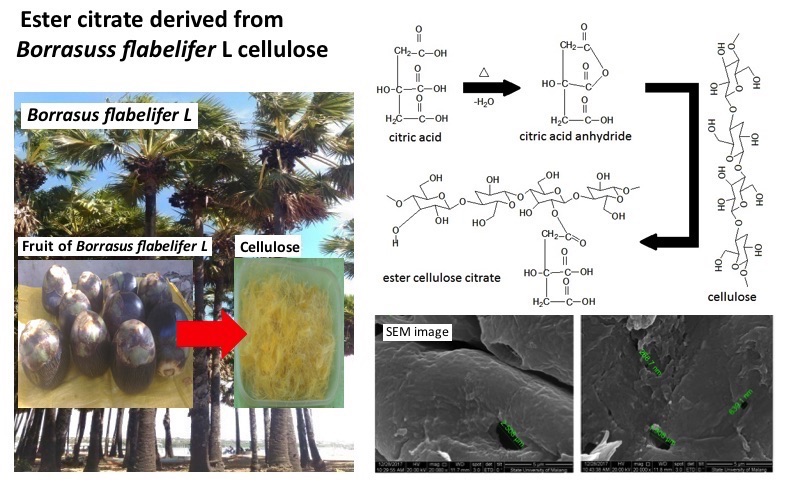
Characteristic of Cellulose Isolated From Papyrus Fibers (Borrasus flabelifer L) And Its Citrate Ester
Abstract
References
[1] Nuroniah, H. S., Rostiawati, T., Bustomi, S., Kosasih, A. K., Syamsuwida, D., Mahfudz, Irawati, S., Pari, G., Sintesis Hasil Penelitian Lontar (Borassus flabellifer) Sebagai Sumber Energi Bioetanol Potensial, Kementrian Kehutanan Badan Penelitian dan Pengembangan Kehutanan, Bogor, 2010.
[2] Boopathi, L., Sampath, P.S., Mylsami, K., Composites:B, 2012, 43, 3044-3052. crossref
[3] Murray, J. C. F., Handbook of Hydrocolloids, Second Edition, 2009, Woodhead Publishing Series in Food Science, Technology and Nutrition, New York.
[4] Perez, S., Samain, D., Adv Carbohydr Chem Biochem, 2010, 64, 3-6. crossref
[5] Grigoryan, G. S., Grigoryan, Z. G., Malkhasyan, A. T., Proceedings of The Yerevan State University, Chemistry and Biology, Obtaining Esters of Citric Acid With High Aliphatic Alcohol, 2017, 51(2), 88-91. website
[6] Low, K. S., Lee, C. K., Mak, S. M., Wood Sci. Technol., 2004, 38, 629-640. website
[7] Firmansyah, D., Rumhayati, B., Masruri., Int. J. ChemTech Res., 2017, 10(4), 674-680. website
[8] Surbakti, S. R., Synthesis Citric Cellulose From Cellulose of Pineapple’s Leaves Through Esterification Reaction With Citric Acid As An Adsorbent On Cadmium Ions (Cd2+). Chemistry Department of Mathematics and Science, Sumatra Utara University, Indonesia, 2016.
[9] Ramirez, J. A. A., Fortunati, E., Kenny, J. M., Torre, L., Foresti, M. L., Carbohydr Polym., 2017, 157, 1358-1364. crossref
[10] Marshall, W. E., Wartelle, L. H., Boler, D. E., Johns, M. M., Toles, C. A., Bioresour Technol, 1999, 69, 263-268. crossref
[11] Genung., Malat, R. C., Ind.Eng.Chem, 1941, 13, 369-374. website
[12] Xu, Y., Miladinov, V., Hanna, M. A., Cereal Chem, 2004, 81(6), 735-740. website
[13] Ma, X., Chang, P. R., Yu, J., Stumborg, M., Carbohydr Polym., 2009, 75, 1-8. website
[14] Roosdiana, A., Mardiana, D., Indahyanti, E., Oktavianie, D. A., RJLS, 2017, 4(1), 18-24. website
[15] Wing, R. E., Ind Crops Prod.,1996, 5, 301-305. crossref
[16] Amini, H. K., Masruri., Ulfa, S. M., J. Pure App. Chem. Res., 2017, 6(2), 93-99. crossref
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.