The Potency of Monoterpenes Contained in Essential Oils of Canary Sap (Canarium indicum L.) as Anti-inflammatory Agent on Asthmatic Rats (Rattus norvegicus)
Abstract
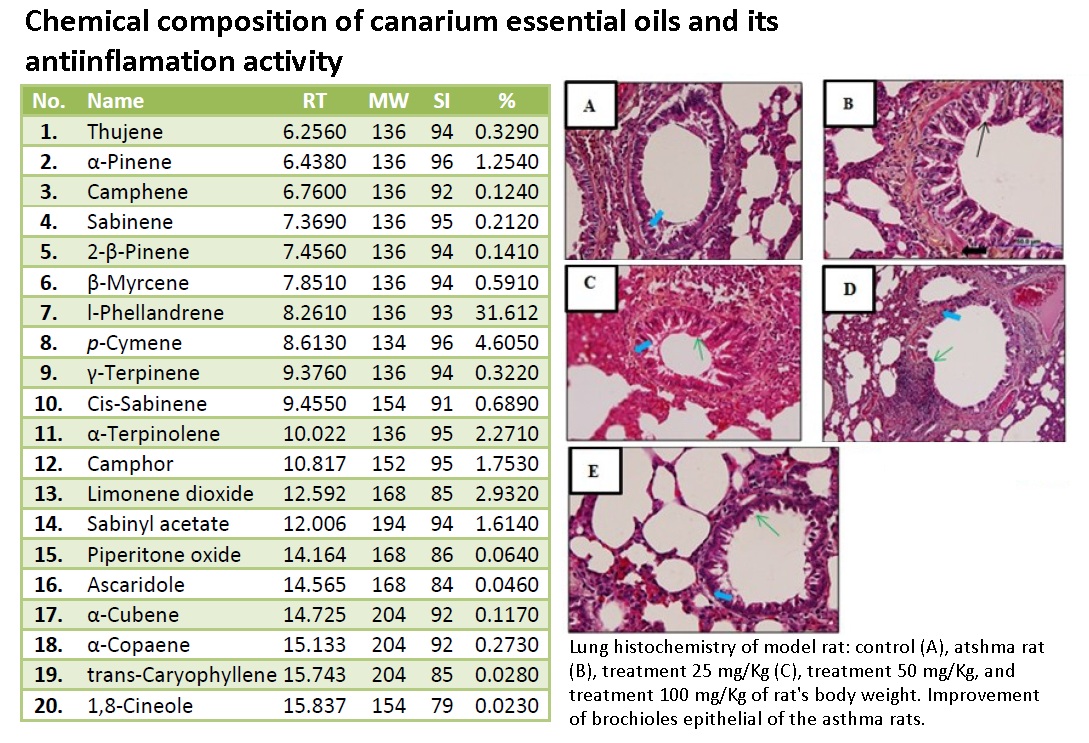
Asthma is a chronic airway disease characterized by significant exacerbation of bronchial spasm (bronchospasm) and airway inflammation. Asthma symptoms become more severe by oral infectious due to exposure of Gram-negative bacterial lipopolysaccharide (LPS). The essential oils (EO) of the canary sap (Canarium indicum L.) contained linalool, p-cymene and γ-terpinene as an anti-inflammatory agent. The aim of this study to determine the potency EO canary therapy toward level of malondialdehyde (MDA) and lung histopathology of asthmatic rats. The asthmatic rats were prepared by sensitization of allergent conducted by intraperitonial injection and nebulized of ovalbumin (OVA) also intrasulcular injection of Lipopolysaccaride from Phorphyromonas gingivalis bacteria. Five groups of rats (Rattus norvegicus) were used in this research; ie the control group, the asthmatic group, and three groups with therapy of EO canary the dose of 25 mg/kg BW, 50 mg/kg BW and 100 mg/kg BW for 7 days. The contain of EO canary were analyzed by Gas chromatography–mass spectrometry (GCMS). The MDA levels were measured using the Thiobarbituric Acid (TBA) technique and histopatology of bronchial stained using Hematoxilin-Eosin (HE) and observed microscopically. The data were analyzed by One Way ANOVA with Tukey test (α = 0.05). The result showed that EO canary significantly (p < 0.05) decrease MDA levels and repair lung histopatology of asthmatic rats. The analysis EO canary performed by GCMS composed of l-phellandrene; p-cymene; γ-terpinene; linalool; camphor; limonene and 1,8-cineole. It can be concluded EO canary has potency as anti-inflammatory agent of asthmatic condition. The most effective dose therapy was obtained as high as 100 mg/kgBW that decreased 46.56 % level of MDA.
References
[1] M. M. Zdanowicz, Am. J. Pharm. Educ., 2007, 71(5), 98.
[2] J. F. Linzer, Clin. Pediatr. Emerg. Med., 2007, 8(2), 87–95.
[3] G. D’Amato, C. Vitale, A. Molino, A. Stanziola, A. Sanduzzi, A. Vatrella, M. Mormile, M. Lanza, G. Calabrese, L. Antonicelli and M. D’ Amato, Multidiscip. Respir. Med., 2016, 11(37), 1–5.
[4] Infodatin, “Prevalensi dan Tata Laksana Asma,” 2017.
[5] T. Janssens and T. Ritz, Clin. Exp. Allergy, 2013, 43(9), 1000–1008.
[6] I. Kucharewicz, Pharmacol. Rep., 2008, 60(1734–1140), 783–788.
[7] H. Utomo, J. Dent. Indones., 2013, 19(3), 57–64.
[8] R. Darveau, A. S, G. I, B. B, and M. R.V, Infect. Immun., 2002, 70(4), 1867.
[9] W. J. Yoon, N. H. Lee, and C. G. Hyun, J. Oleo Sci., 2010, 59(8), 415–421.
[10] A. Tang, A. Sharma, R. Jen, A. F. Hirschfeld, M. A. Chilvers, P. M. Lavoie, S. E. Turvey, PLoS ONE, 2012, 7(5), 1–12.
[11] J. Y. Cho, M. Miller, K. J. Baek, J. W. Han, J. Nayar, S. Y. Lee, K. McElwain, S. McElwain, S. Friedman, and D. H. Broide, J. Clin. Invest., 2004, 113(4), 551.
[12] J. Palomo, D. Dietrich, P. Martin, G. Palmer, and C. Gabay, Cytokine, 2015, 76(1), 25–37.
[13] A. A. Fatriyawan, C. Mahdi, A. Aulanni’am, and D. K. Wuragil, Int. J. ChemTech Res., 2016, 9( 4), 509–512.
[14] D. K. Wuragil and A. Aulanni’am, J. Appl. Sci. Res., 2012, 8(11), 5311–5316.
[15] Z. Singh, I. P. Karthigesu, P. Singh, and R. Kaur, J. Public Health, 2014, 43(3), 7.
[16] J. F. Vasconcelos et al., Int. Immunopharmacol., 2008, vol. 8, no. 9, pp. 1216–1221.
[17] K. Kiptiyah, W. Widodo, G. Ciptadi, M. A. Widodo, and S. B. Sumitro, J. Taibah Univ. Med. Sci., 2017, 12(5), 397–406.
[18] J. J. W. Coppen, Gums, resins and latexes of plant origin, 1995, Food and Agriculture Organization of the United Nations, Rome.
[19] R. Muthuswamy and S. R, IJPS, 2013, 9(2), 13–21.
[20] D. Meena, N. Binaibabu, and J. Doss, Int. J. Conserv. Sci., 2012, 3(3).
[21] S. Muniraj, T. Nanthakumaran, S. Shanthasubitha, and B. Shanmugapriya, IAJMR, 2016, 2, 484–493.
[22] P. M. Giang, W. A. Konig, and P. T. Son, Chem. Nat. Compd., 2006, 42(5), 523–524.
[23] R. de Cássia da Silveira e Sá, L. Andrade, and D. de Sousa, Molecules, 2013, 18(1), 1227–1254.
[24] C. D. Pusparini, Aulanni’am, and D. A. Oktavianie, Program Studi Pendidik. Dr. Hewan Univ. Brawijaya, 2012.
[25] J. A. Chapman, I. L. Bernstein, R. E. Lee, and J. Oppenheimer, Allergy Asthma Immunol., 2005, 96.
[26] L. Putu Gina, A. Aulanni’am, and C. Mahdi, J. Pure Appl. Chem. Res., 2016, 5(1), 40–47.
[27] R. Mogana and C. Wiart, J. Pharm. Res., 2011, 4(8), 2482–2489.
[28] H. D. S. Siqueira, B. S. Neto, D P.Sousa, B. S. Gomesa, F. V. Silva, F. V.M. Cunha, C. W. S. Wanderley, G. Pinheiro, A. G. F. Cândido, D. V. T. Wong, R. A.Ribeiro, R. C. P. Lima-Júnior, F. A. Oliveira, Life Sci., 2016, 160, 27–33.
[29] A. Ayala, M. F. Muñoz, and S. Argüelles, Oxid. Med. Cell. Longev., 2014, 2014, 1–31.
[30] R. Mitra, S. Datta, M. Pal, K. Ghosh, D. Paul, and K. Pal, Br. J. Med. Med. Res., 2015, 9(7), 1–7.
[31] I. Bara, A. Ozier, J.-M. Tunon de Lara, R. Marthan, and P. Berger, Eur. Respir. J., 2010, 36(5), 1174–1184.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








