Synthesis of Patchouli Biochar Cr2O3 Composite Using Double Acid Oxidators for Paracetamol Adsorption
Abstract
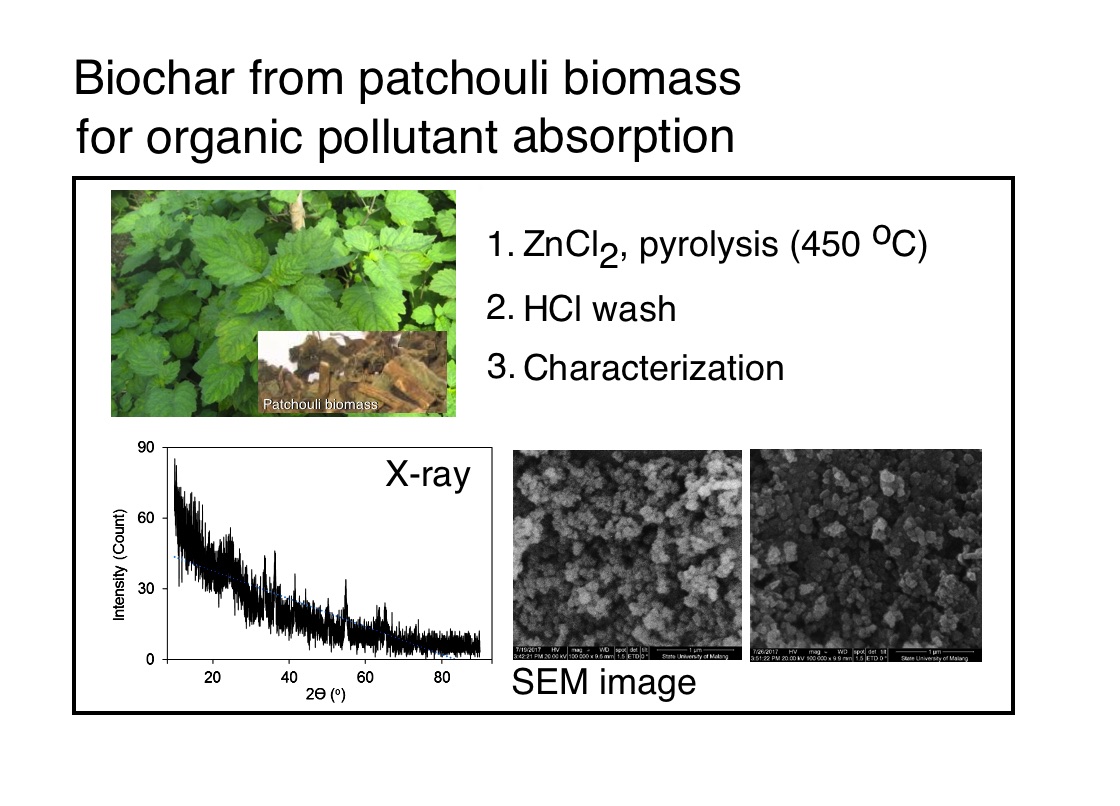
Composite built by patchouli biochar and metal oxide, Cr2O3, is a potential material for remediation of contaminated wasterwater. Oxidation of biochar using acid or salt oxidators can improve its surface polar functional groups. This treatment may be able to increase impregnation of metal cation (as salt) before calcination to form its oxide. In this research, 3 types of oxidators were used to oxidize the biochar before impregnation with purpose to study its influence toward physichochemistry and adsorption performance of the composite. Preparation of the composite included 3 steps, including preparation of biochar by pyrolisis of patchouli biomass using ZnCl2 activator at 450 oC, oxidation of the biochar using 3 different oxidators (H2SO4-HNO3, H3PO4-HNO3, H2O2–HNO3) at 60 oC, impregnation of the oxidized biochar using CrCl3 followed by calcination process to form biochar–Cr2O3 composite at 600 oC. Characterization using X-ray diffraction indicated that the composite containes the Cr2O3 structure. FTIR spectrophotometry characterization indicates the different content of C=O, C-O, and –OH on the composite surface. SEM images shows irregular micro ball shapes. EDX characterization indicates the different Cr content in the composite with same sequence with FTIR absorbances of both C-O and –OH. Adsorption of paracetamol indicates effect of Cr2O3 showing the same sequence of both.
References
[1] | Heckley, E., The Structural Changes of Hydrothermally Treated Biochar Caused by Ball-Milling, Juan Mauricio Venegas, 2014. website |
[2] | Klinsmann Ng Weng Sum, Adsorption of Trimethyltin, Arsenic (v), Zinc and Copper by Palm Oil Mill Sludge Biochar Prepared by Microwave, Faculty of Engineering and Green Technology, University Tunku Abdul Rahman, 2016. website |
[3] | Fang, C., Zhang, T., Li, P., Jiang, R., and Wang, Y., Int. J. Environ. Res. Public Health, 2014, 11, 9217-9237. website |
[4] | Jung, C., Phal, N., Oh, J., Chub, KH., Jang, M., Yoon, Y., J Hazard Mater, 2015, 300, 808–814. crossref |
[5] | B. Peng, L. Chen, C. Que, K. Yang, F. Deng, X. Deng, G. Shi, G. Xu, M. Wu, Sci. Rep. 2016, 6(31920), 1-10. website |
[6] | Setianingsih, T., Ismuyanto, B., Masruri, Int J Chemtech Res., 2016, l9(12), 610-621. |
[7] | Moosavi, E., Dastgheib, S., and Karimzadeh, R., Energies., 2012, 5, 4233-4250 |
[8] | Han, Z., Sani, B., Mrozik, W., Obst, M. Beckingham, B., Karapanagioti, HK., Werner, D., Water Res., 2015, 70(1), 394-403 |
[9] | Shen, W., Li, Z., and Liu, Y., Recent Patents on Chemical Engineering, 2008, 1, 27-40 |
[10] | Kulkarni, S., JCCS, 2015, 5(8), 443-447 |
[11] | Olivier, C.F., An investigation into the degradation of biochar and its interactions with plants and soil microbial community, Faculty of AgriSciences, Stellenbosch University, 2011 |
[12] | Yakout, S.M., Daifullah, A.H.M., el-reefy, S.A., EEMJ, 2015, 14( 2), 473-480 |
[13] | Ahmed, DS., Haider, AJ., Mohammad, MR., Energy Procedia, 2013, 36, 1111 – 1118 |
[14] | Moreno-Castilla, C., Carbon, 2004, 42, 83–94 |
[15] | Atieh, M.A., IJESD, 2011, 2(2), 1-4 |
[16] | Lawrinenko, M., Anion exchange capacity of biochar, Iowa State University, 2014 |
[17] | Farzaneh, F. and Najafi, M., J. Sci. I. R. Iran, 2011, 22(4), 329-333 |
[18] | Abbas, A., Abussaud, B.A., Ihsanullah, Al-Baghli, N.A.H., Khraisheh, M., and Atieh, M.A., J Nanomater., 2016, 2016, 1-10. |
[19] | Park, J., Kim, G. P., Nam, H.N.U.I., Park, S., Kim, Y., Yi, J., J Nanopart Res, 2013, 15(1943), 1-9. |
[20] | Moreno-Piraján, J.C., Tirano, J., Salamanca, B., and Giraldo, L., Int. J. Mol. Sci. 2010, 11, 927-942. |
[21] | Hassen, A, El Sayed, M, Morsi, W.M. and El-Sayed, S., J. Appl. Phys., 2012, 112, 093525-1-8. |
[22] | Cellard, A., Zenati, R., Garnier, V., Fantozzi, G., Baret, G., J. Eur. Ceram. Soc., 2007, 27, 1017-1021. |
[23] | Sonea, B.T., Manikandana, E., Gurib-Fakima, A., and Maazaa, M., Green Chem. Lett. Rev., 2016, 9(2), 85–90. |
[24] | Wu, J., 2004, Modeling Adsorption of Organic Compounds on Activated Carbon A multivariate approach, Université de Neuchâtel, Neuchâtel, Schweiz, 2004. |
[25] | Oscik, J., Cooper, I.L (editor), Adsorption, 1982, John Wiley & Sons Inc., Queensland. |
[26] | Windolz, M., Budavari, S., Blumeti,RF., Otterbein, ES., The Merc Index : An Encyclopedia Of Chemicals, Drugs, and Biologicals, 10th ed, 1983, Merck & CO, Inc., USA. |
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








