Modification of Screen Printed Carbon Electrode (SPCE) with Polypyrrole (Ppy)-SiO2 for Phenol Determination
Abstract
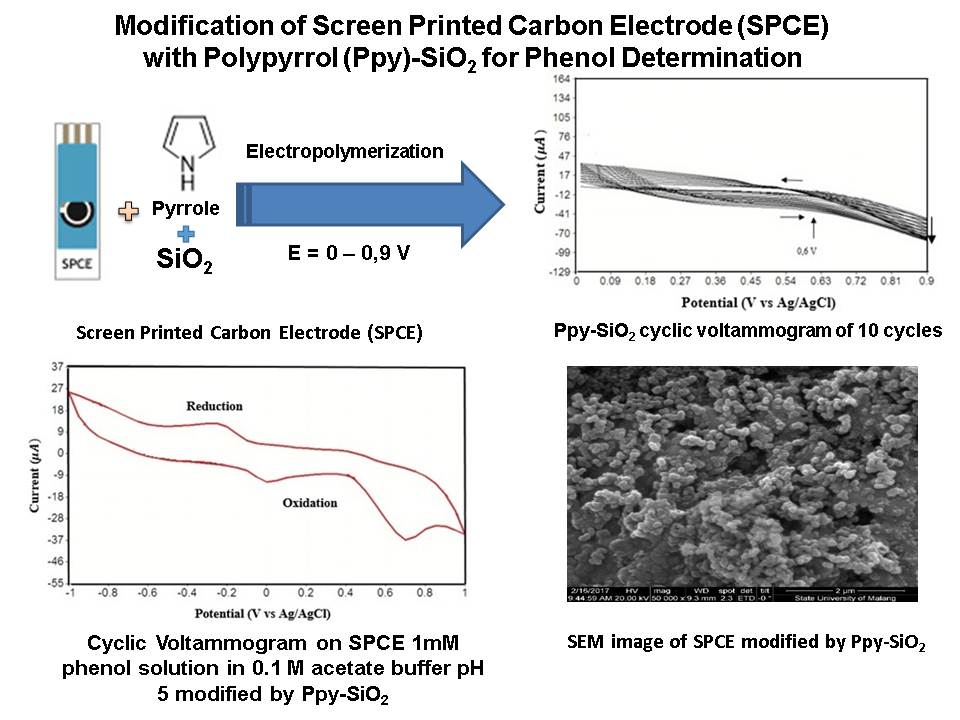
References
[1] Mojovic, Z., Jovic-Jovicic, N., Bankovic, P., M. Žunić A., Abu Rabi-Stanković, A. Milutinović-Nikolić, D. Jovanović, Appl Clay Sci., 2011, 53(2), 331-335. crossref
[2] Dewilda, Y., R. Afrianita and F. F., Jurnal Teknik Lingkungan, 2012, 9, 59-73.
[3] Republik Indonesia, Peraturan Pemerintah Republik Indonesia Nomor 3 Tahun 2010 Baku Mutu Air Limbah Bagi Kawasan Industri, 18 Januari 2010, Lembaran Negara Republik Indonesia Tahun 2010 Nomor 3, Jakarta, 2001.
[4] Z. Yueming, W. Haiyun, Y. Wei, C. Chuanjin., and W. Xiaowen, Sensors, 2013, 13, 8551-8563. website
[5] Renedo, O. D., Alonso-Lomillo, M.A. dan Martinez, M.J.A., Talanta, 2007, 73, 202-219. website
[6] T. El Ouafy, A. Chtaini, H. Oulfajrete, R. Najih, LEJPT, 2014, 25, 166-178. website
[7] Alemu, H., Negash, N.,Tessema, M. and Al, E. T. Anal. Chem., 2014, 5, 188–198. website
[8] Negash, N., Alemu, H. and Tessema, M., Int. Schol. Res. Not. 2015, 2015, 1-11. crossref
[9] Kim, B. C. The Synthesis and Characterisation of Hydrogel and Polypyrrole Blends. Thesis, University of Wollongong, 1999. website
[10] Sadki, S., Schottland, P., Brodiec, N., and Sabouraud, G. Chem Soc Rev, 2000, 29, 283–293. website
[11] Kamalzadeh, Z., and Shahrokhian, S. Bioelectrochemistry, 2014, 98, 1–10. crossref
[12] Z. Min, Y. Wu, X. Feng, X. He, L. Chen and Y. Zhang. J. Mater. Chem., 2010, 20, 5835-5842. website
[13] A. Mulyasuryani, Ngibad, K. and Mardiana, D. Alchemy, 2016, 12, 36-49. crossref
[14] A Wanekaya, O. A. Sadik, J. Electroanal. Chem., 2002, 537, 135–43. crossref
[15] Xie, A., Wu, F., Xu, Z. and Wang, M. Compos. Sci. Technol, 2015, 117, 32–38. crossref
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








