Studies of In Vitro and In Silico of Immobilized Xylanase on Zeolite Matrix Activated with Hydrochloric Acid
Abstract
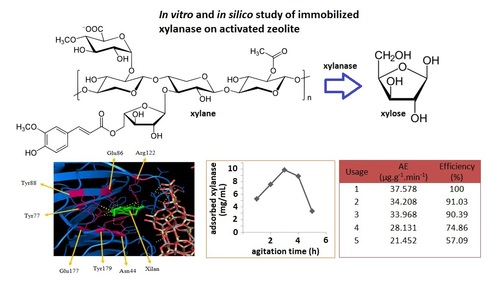
Xylanase is a hydrolase enzyme that can hydrolyze hemicellulose into xilo-oligosaccharide and xylose. This research is aimed to investigate the in vitro and in silico xylanase (isolated from the fungus Trichoderma viride) immobilized on the zeolite matrix activated with HCl 0.4 M solution. The study was performed using in silico docking molecular methods to investigate the interaction between the xylanase primarily to its ligand. Xylanase activity was determined by reducing sugar produced (xylose) by 1 mL of enzyme per minute. The optimum conditions of immobilized xylanase were measured according to the time agitation and concentration of xylanase. The time variation agitation used were 1, 2, 3, 4, and 5 hours, while variations in the concentrations of a xylanase used were 11.500, 15.653, 20.444, 25.875, and 31.944 mg/mL. The optimum conditions of immobilized xylanase was obtained in the shaking time for 3 hours at a concentration of xylanase 15.653 mg/mL and immobilized xylanase activity generated at 46.755 μg.g-1.min-1. Immobilized xylanase activity was greater than the purified xylanase (15.976 μg.mL-1.min-1). These results were due to the cofactors Al (AlO4) and Si (SiO4) of zeolite was able to increase the kinetic energy caused the reaction rate between xylanase with the larger substrates. Cofactor also increased the kinetic energy and can enhance the rate of reaction between a xylanase with its substrate, in order to give greater activity. Immobilized xylanase was stable and its reusability as much as 6 times which afforded the activity 21.331 μg.g-1.min-1 and the efficiency of 56.77%.
References
[1] Poedjiadi, A. dan Supriyanti F. M. T., Dasar-Dasar Biokimia, 2006, UI-Press, Jakarta.
[2] Konanki, S., Jayasimha. R. D., Anitha. S. and Muralidhararao. D., IJPAES, 2013, 4(10), 19–24
[3] Richana, N., T. Irawadi, N. Anwar dan K. Syamsu, Jurnal Agro Biogen, 2008, 4(1), 24- 34
[4] Megazyme, endo-1,4-β-xylanase M1 (from Trichoderma viride) (20502), http://secure.megazyme.com/files/BOOKLET/E-XYTR1_020502_DATA.pdf, 2009, accessed date : 5 April 2016
[5] Nurlaili, N., Uji Biologis Bungkil Biji Jarak Pagar (Jatropha curcas L.) yang Diolah dengan Ekstrak Metanol dan Fermentasi menggunakan Rhizopus oryzae serta Trichoderma viride pada Ayam Broiler, Skripsi, Departemen Ilmu Nutrisi dan Teknologi Pakan Fakultas Peternakan IPB, Bogor, 2009
[6] Eugenia, Sesan T. and Oancea F., J. Plant Develop., 2010, 17, 49-62
[7] Widjaja, S., T. Purwadaria dan P.P. Ketaren, Microbiol Indones, 2008, 2, 44-48
[8] Hiratsuka, A., Fujisawa K., and Muguruma H., Anal Sci, 2008, 24, 483–486
[9] Sheldon, Roger A., Adv. Synth. Catal., 2007, 349, 1289-1307
[10] Akimkhan, A. M., Structural and Ion-Exchange Properties of Natural Zeolite, Lisence in tech, 2012, pp. 261-282
[11] Bogdanov, B., D. Georgiev., K. Angelova,.and Y. Hristov, Synthetic Zeolites and Their Industrial and Environmental Applicat Ions Review. International Science conference. Volume IV Natural & Mathematical science, 2009
[12] Lailah, N., Prasetyawan, S., and Srihardyastutie, A., J. Pure App. Chem. Res., 2017, 6(2), 150-159
[13] Noolvi, M. N., and Patel, H. M., J. Saudi Chem. Soc., 2013, 17, 361 – 379
[14] Zhang, D., J. Ai, Z. Liang, C. Li, X. Peng, Y. Ji, H. Jiang, M. Geng, C. Luo, H. Liu, Bioorg. Med. Chem., 2012, 20, 5169 – 5180.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








