Investigation of the Electronic and pH-Sensing properties of Hydroxyl-Functionalized Imine-Linked Polymers via the UV-vis Absorption Spectra and the Density Functional Theory (DFT) Calculations
Abstract
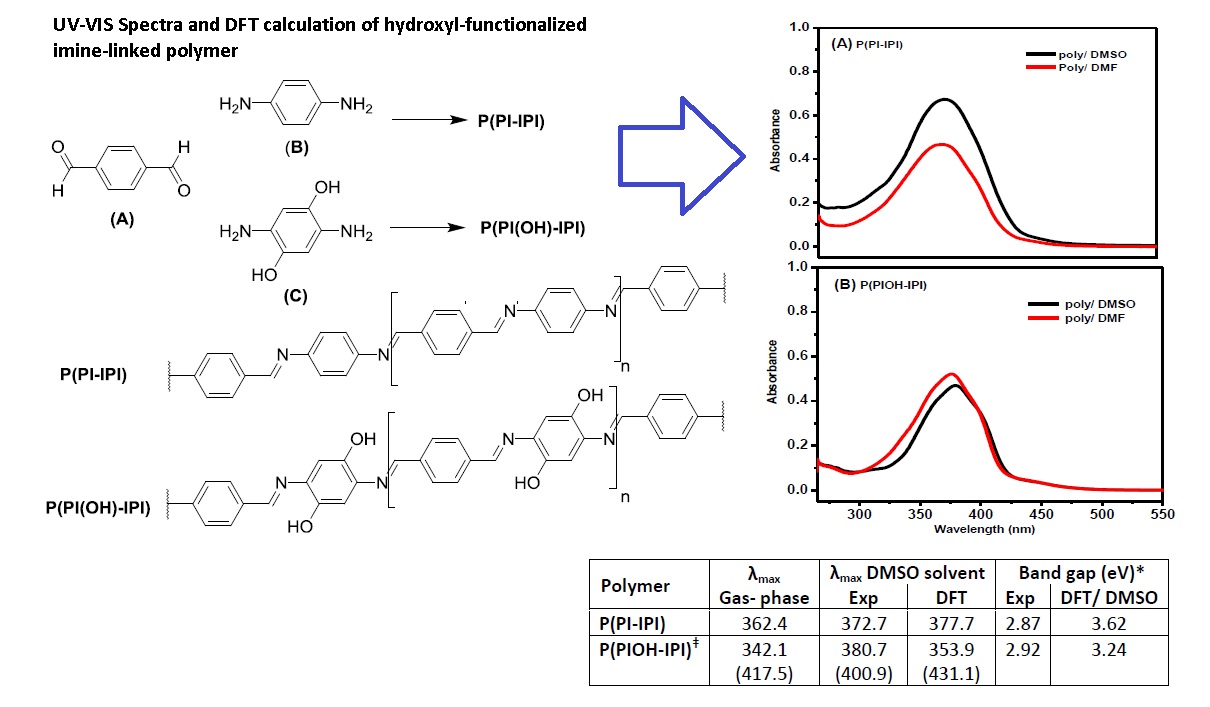
In this report, a synergetic computational and experimental studies were demonstrated on examples of poly-imine polymers; P(PI-IPI) and P(PIOH-IPI) to explore the role of hydroxyl substituent on their sensing and electronic properties. The polymer P(PIOH-IPI) bearing the OH-group on the ortho-position to the imine-bond, while the structure of the polymer P(PI-IPI) reveal the imine-bond only. The sensing property of the polymers was investigated via the UV-vis absorption in different solvents, acidic and basic solutions. Both polymers have shown significant sensing behavior in the acidic medium, while unpronounced behavior was noticed in the case of the polymer P(PI-IPI) in basic medium. Upon the incorporation of the OH-group, the polymer P(PIOH-IPI) has indistinguishable sensing behavior, a similar blue-shift in the acidic and basic medium, which can be attributed to the presence and the position of OH-group. The optical band gap of the polymers was determined experimentally and theoretically from the UV-vis absorption spectra and DFT calculations in the DMSO solvent. Other factors that affect the band gap values such as the structural conformation and length of conjugation were explored theoretically. In general, as the length of the optimized chain increased, the spectrum is red-shifted and the band gap decreased, which is attributed to the possible loss of chain planarity and conjugation beyond the monomer structure. Interestingly, the UV-vis spectra of the monomer-optimized structures were in a good match with the experimental UV-vis spectra. However, the band gap difference can be attributed to the method of band gap determination.
References
[1] Li, J., Liu, J., Wei, C. W., Liu, B., O'Donnell, M. and Gao, X, Phys Chem Chem Phys, 2013, 15 (40), 17006-15.
[2] Jung, J. W., Jo, J. W., Chueh, C. C., Liu, F., Jo, W. H., Russell, T. P., and Jen, A. K. Y, Adv. Mater, 2015, 27 (21), 3310-3317.
[3] Guifu, C., F. Wang, Y. Wang, X. Zhang, H. Qin, H. Zou, J. Xu, Chinese. J. Catal., 2014, 35 ( 4), 540-545.
[4] Facchetti, A., Chem. Mater, 2011, 23 (3), 733-758.
[5] Dutta, P. K., Jain, Pragya, Sen, Pratima, Trivedi, Rashmi, Sen, P. K. and Dutta, Joydeep, Eur. Polym. J, 2003, 39 ( 5), 1007-1011.
[6] Cheng, Y. J., Yang, S. H. and Hsu, C. S., Chem. Rev, 2009, 109(11), 5868-5923.
[7] Chang, C. C., Pai, C. L., Chen, W. C. and Jenekhe, S. A, Thin Solid Films, 2005, 479 (1–2), 254-260.
[8] Abu-Dief, A. M., I. M. A. Mohamed, Beni-Suef Univ. J. Appl. Sci., 2015, 4(2), 119-133.
[9] Supriadi, M. B., Chen, M. C., Li, Y. S., J. Pure. App. Chem. Res., 2013, 2(1), 1-10.
[10] Verde-Sesto, Ester, Maya, Eva M., Lozano, Angel E., de la Campa, Jose G., Sanchez, Felix and Iglesias, Marta, J. Mater. Chem, 2012, 22(47), 24637-24643.
[11] Ding, S. Y., Gao, J., Wang, Q., Zhang, Y., Song, W. G., Su, C. Y. and Wang, W., J. Am. Chem. Soc., 2011, 133(49), 19816-19822.
[12] Mondal, S. and Das, N., J. Mater. Chem. A, 2015, 3 (46), 23577-23586.
[13] Adhikari, B. and Majumdar, S., Prog Polym Sci, 2004, 29 ( 7), 699-766.
[14] Koyuncu, S., Kaya, İ., Koyuncu, F. B. and Ozdemir, E., Synthetic Met., 2009, 159 (11), 1034-1042.
[15] Lamarque, T., Le Barny, P., Obert, E., Chastaing, E., Loiseaux, B. and Leray, I, Proc. SPIE, Optical Sensing II, 2006, 6189, 1-12.
[16] Sharma, H., Kaur, N., Pandiyan, T. and Singh, N., Sens Actuators B Chem., 2012, 166–167, 467-472.
[17] Wang, J., Senkovska, I., Oschatz, M., Lohe, M. R., Borchardt, L., Heerwig, A., Liu, Q. and Kaskel, S., ACS Appl Mater Interfaces, 2013, 5 (8), 3160-7.
[18] Altarawneh, S. S., Jazzazi, T., Ababneh, T. S, Al Shboul, T. M. A. and Al Jaafreh, I. Y, J. Appl. Polym. Sci., 2017, 134(2), 1-9.
[19] Xu, L., Cao, L., Guo, Z., Zha, Z. and Lei, S., Chem. Comm, 2015, 51 (41), 8664-8667.
[20] Patil, S., Jadhav, S. D. and Shinde, S. K, Org Chem Int, 2012, 2012, 1-5.
[21] Berkesi, O., Kortvelyesi, T., Hetenyi, C., Nemeth, T. and Palinko, I., Phys Chem Chem Phys, 2003, 5 (10), 2009-2014.
[22] Rabbani, M. G., Sekizkardes, A. K., Kahveci, Z., Reich, T. E., Ding, R. and El-Kaderi, H. M, Chem. -Eur. J, 2013, 19(10), 3324-3328.
[23] Altarawneh, S., Behera, S., Jena, P. and El-Kaderi, H. M, Chem. Comm, 2014, 50(27), 3571-3574.
[24] Iwan, A. and Rannou, P, Spectrochim Acta A, 2009, 74(1), 174-9.
[25] Debnath, D., Roy, S., Li, B. H., Lin, C. H. and Misra, T. K., Spectrochim Acta A, 2015, 140, 185-197.
[26] Kumbul, A., Gokturk, E., Turac, E. and Sahmetlioglu, E., Polym. Adv. Technol, 2015, 26 ( 9), 1123-1129.
[27] Gündüz, B., J. Appl. Polym. Sci., 2015, 132(11), 1-8.
[28] Gündüz, B., Optik, 2015, 126 (23), 4566-4573.
[29] Knöpke, L. R., Spannenberg, A., Brückner, A. and Bentrup, U., Spectrochim Acta A, 2012, 95, 18-24.
[30] Kosar, B., Albayrak, Ç., Odabaşoğlu, M. and Büyükgüngör, O., J. Mol. Struc, 2011, 989 (1–3), 31-37.
[31] Kumbul, A., Gokturk, E. and Sahmetlioglu, E., J Polym Res, 2016, 23 (3), 1-11.
[32] M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, Al M. A. Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 09, Revision B.01, 2010, Gaussian, Inc., Wallingford CT.
[33] Bardak, F., Karaca, C., Bilgili, S., Atac, A., Mavis, T., Asiri, A. M., Karabacak, M. and Kose, E. Spectrochim Acta A, 2016, 165, 33-46.
[34] Rittmeyer, S. P. and Groß, A, Beilstein J Nanotechnol, 2012, 3, 909-19.
[35] Botelho, AéLã, Shin, Y., Liu, J. and Lin, X, PLoS One, 2014, 9 (1), 1-8.
Refbacks
- There are currently no refbacks.









