Absorption Activity of Cassava Peel (Manihot utilissima) as Chromium (VI) Metal Biosorbent in Electroplating Waste
Abstract
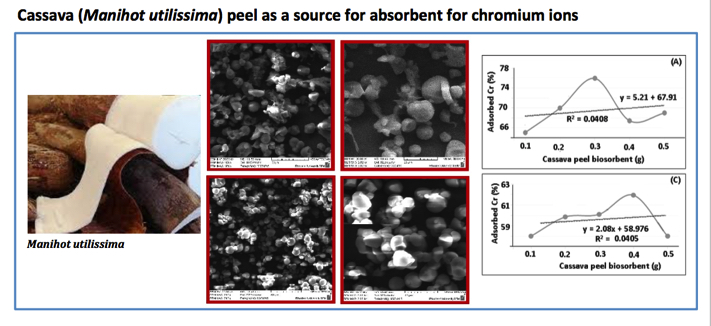
Electroplating is a process of metal veneering with another metal using the electric energy. The water waste of electroplating industry contains many kinds of heavy metal ions, especially chromium (VI) which might cause pollutions if it was not processed and it presents above the threshold allowed. The use of cellulose can be a solution, because it has the functional groups which form bonding with the metal ions. Cassava peel is one of the sources of cellulose which contains 80-85% of cellulose. This proves that cassava peel (Manihot utilissimaI) has the potential as the heavy metal biosorbent of chromium metal in electroplating waste. The methodology of the research is conducted in a series including analysis of heavy metal concentrations of chromium (VI) in electroplating waste. Biosorption treatment of cassava peel (Manihot utilissimaI) biosorbent activated by HNO3 1.5 M in electroplating waste conducted in the batch method. Analysis of heavy metal concentrations of chromium (VI) in electroplating waste was performed by Atomic Absorption Spectrophotometry (AAS) after biosorbtion process. Variation of biosorbent mass were (0.1, 0.2, 0.3, 0.4, 0.5) grams, and variation of biosorbent contact time were (10, 20, 30, 40, 50) minutes. The analysis result recorded by AAS (Atomic Absorption Spectrophotometry) showed that the level of total chromium in electroplating waste reaches 2.0777 ± 0.2785 ppm. Accordingly, the chromium test solution used in this research was 2 ppm to measure the optimum conditions of % chromium (VI) adsorbed with variation of mass and contact time. From the results of this research, the optimum mass and contact time of cassava peel biosorbent activated by HNO3 1.5 M in % chromium (VI) adsorbed were 0.1 gram and 40 minutes, respectively. Finally, the optimum mass and contact time of cassava peel biosorbent activated by HNO3 1.5 M has applied to electroplating waste. The % chromium adsorbed in electroplating waste with the addition of cassava peel biosorbent activated by HNO3 1.5 M was measured in average of 61.72%.
References
[1] Diantariani, N. P., I. W. Sudiarta, dan N. K. Elantiani, JKIM, 2008, 2(1), 45-52.
[2] Drake, Thelbert, L., William, H., Roe, The Principalship, 1996, London, Mac Millan Pub.
[3] Ronda, A., Martin-Lara, M. A., Calero, M., Blazquez, G., Ecol. Eng., 2013, 58, 278–285.
[4] Herwanto, B. dan Santoso, E., Akta Kimindo, 2006, 2(1), 9-24.
[5] Ibbet, R. N., Kaenthong, S., Philips, D. A. S., Wilding, M. A., Lenzinger Ber, 2006, 88, 77-86.
[6] Saefudin and A. Z Raziah, J. Appl. Sci. Res., 2007, 3(12), 2091-2099.
[7] Parvathi, K., Nagendran, R., and Narehkumar, R., Electron. J. Biotechnol., 2007, 10(1), 92-105.
[8] Baral, S. S., Das, N., Chaudhury, G. R., Das, S. N., J. Hazard. Mater., 2009, 171, 358–369.
[9] Rios J. P., Bess-Oberto L., Tiemann K. J. and Gardea-Torresdey. Proceedings of the 1999 Conference on Hazardous Waste Research, University of Texas at El Paso, El Paso, 1999.
[10] M. A. Javed, H. N. Bhatti, M. A. Hanif, R. Nadeem, Sep. Sci. Technol., 2007, 42(16), 3641–3656.
[11] B. Greene, Mcpherson, D. W. Darnall, Algal sorbents for selective metal ion recovery, in J. Patterson, R. Pasino (Eds.), Metal Speciation, Separation and Recovery, 1987 Lewis Publisher, Michigan.
[12] K. K. Pandey, G. Prasad, V. N. Singh, J. Chem. Technol. Biotechnol., 1984, 34(7), 367–374.
[13] Karthikeyen, T., Rajgpal, S., Miranda, L. R., J. Hazard. Mater., 2005, 124, 192–199.
[14] N. Kannan, T. Srinivasan, Natural adsorbents for water and wastewater treatment: a review, in: R.K. Trivedy (Ed.), Eco Technologies for Pollution Control and Environmental Management, 1997, Tata McGraw Hill Publ., New Delhi.
[15] K. Mohanty, M. Jha, B. C. Meikap, M. N. Biswas, Chem. Eng. J., 2006, 117, 71–77.
[16] M. Viswanadham, N. Sriramula, M. C. Adharvana, Indian J. Environ. Prot. 2000, 20, 515–520.
[17] R. Saravanane, T. Sundararajan, S. Sivamurthyreddy, Indian J. Environ. Health, 2002, 44, 78–81.
[18] W. E. Marshall, E. T. Champagne, J. Environ. Sci. Health, 1995, 30, 241–261.
[19] Ramrakhiani, L., Halder, A., Majumder, A., Mandal, A. K., Majumdar, S., Ghosh, S. Chem. Eng. J., 2017, 308, 1048–1064.
[20] Ronda, A., Calero, M., Blázquez, G., Pérez, A., Martín-Lara, M. A., J Taiwan Inst Chem Eng, 2015, 51, 109–118.
[21] Abdolali, A., Ngo, H. H., Guo, W., Zhou, J. L., Du, B., Wei, Q., Wang, X. C., Nguyen, P. D., Bioresour. Technol., 2015, 193, 477–487.
[22] Liu, C., Fiol, N., Poch, J., Villaescusa, S., JWPE, 2016, 11, 143–151.
[23] Rezaei, Hassan, Arab. J. Chem., 2016, 9, 846–853.
[24] Daud, Z., Kassim, A. S. M., Aripin, A. M., Awang, H., Hatta, M. Z. M., Aust. J. Basic & Appl. Sci, 2013, 7(9), 406-411.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








